Suramin inhibits renal fibrosis in chronic kidney disease
- PMID: 21617121
- PMCID: PMC3103726
- DOI: 10.1681/ASN.2010090956
Suramin inhibits renal fibrosis in chronic kidney disease
Abstract
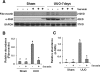
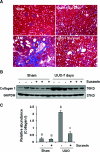
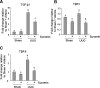
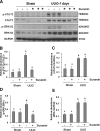
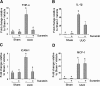
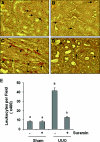
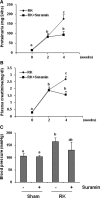
The activation of cytokine and growth factor receptors associates with the development and progression of renal fibrosis. Suramin is a compound that inhibits the interaction of several cytokines and growth factors with their receptors, but whether suramin inhibits the progression of renal fibrosis is unknown. Here, treatment of cultured renal interstitial fibroblasts with suramin inhibited their activation induced by TGF-β1 and serum. In a mouse model of obstructive nephropathy, administration of a single dose of suramin immediately after ureteral obstruction abolished the expression of fibronectin, largely suppressed expression of α-SMA and type I collagen, and reduced the deposition of extracellular matrix proteins. Suramin also decreased the expression of multiple cytokines including TGF-β1 and reduced the interstitial infiltration of leukocytes. Moreover, suramin decreased expression of the type II TGF-β receptor, blocked phosphorylation of the EGF and PDGF receptors, and inactivated several signaling pathways associated with the progression of renal fibrosis. In a rat model of CKD, suramin abrogated proteinuria, limited the decline of renal function, and prevented glomerular and tubulointerstitial damage. Collectively, these findings indicate that suramin is a potent antifibrotic agent that may have therapeutic potential for patients with fibrotic kidney diseases.
Figures





References
-
- Neilson EG: Mechanisms of disease: Fibroblasts—A new look at an old problem. Nat Clin Pract Nephrol 2: 101–108, 2006 - PubMed
-
- Boor P, Ostendorf T, Floege J: Renal fibrosis: Novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 6: 643–656, 2010 - PubMed
-
- Grande MT, Lopez-Novoa JM: Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat Rev Nephrol 5: 319–328, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

