Knockout of toll-like receptor-2 attenuates both the proinflammatory state of diabetes and incipient diabetic nephropathy
- PMID: 21617141
- PMCID: PMC3148786
- DOI: 10.1161/ATVBAHA.111.228924
Knockout of toll-like receptor-2 attenuates both the proinflammatory state of diabetes and incipient diabetic nephropathy
Abstract
Objective: Type 1 diabetes (T1DM) is a proinflammatory state and confers an increased risk for vascular complications. Toll-like receptors (TLR) could participate in diabetic vasculopathies. Whether TLR activation contributes to the proinflammatory state of T1DM and the pathogenesis of diabetic nephropathy remains unknown.
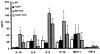
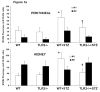
Methods and results: We induced T1DM in TLR2 knockout mice (TLR2-/-) and wild-type littermates (C57BL/6J-WT) using streptozotocin (STZ). Fasting blood, peritoneal macrophages, and kidneys were obtained for flow cytometry, Western blot, microscopy, and cytokine assays at 6 and 14 weeks after induction of diabetes. Macrophage TLR2 expression and MyD88-dependent signaling were increased in diabetic mice (WT+STZ) compared with nondiabetic WT mice. These biomarkers were attenuated in diabetic TLR2-/- macrophages. WT+STZ mice showed increased kidney:body weight ratio due to cell hypertrophy, increased albuminuria, decreased kidney nephrin, podocin, and podocyte number and increased transforming growth factor-β and laminin compared with WT mice. Nephrin, podocin, and podocyte number and effacement were restored, and transforming growth factor-β and laminin levels were decreased in TLR2-/-+ STZ mice kidneys versus WT+STZ. Peritoneal and kidney macrophages were predominantly M1 phenotype in WT+STZ mice; this was attenuated in TLR2-/-+STZ mice.
Conclusions: These data support a role for TLR2 in promoting inflammation and early changes of incipient diabetic nephropathy, in addition to albuminuria and podocyte loss.
Figures







References
-
- Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, Hsueh W, Rewers M, Roberts BT, Savage PJ, Skarlatos S, Wassef M, Rabadan-Diehl C. National Heart, Lung, and Blood Institute; National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation. 2005;111:3489–3493. - PubMed
-
- Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55:774–779. - PubMed
-
- Schram MT, Chaturvedi N, Schalkwijk CG, Fuller JH, Stehouwer CD EURODIAB Prospective Complications Study Group. Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes--the EURODIAB Prospective Complications Study. Diabetologia. 2005;48:370–378. - PubMed
-
- Schalkwijk CG, Poland DC, van Dijk W, Kok A, Emeis JJ, Dräger AM, Doni A, van Hinsbergh VW, Stehouwer CD. Plasma concentration of C-reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation. Diabetologia. 1999;42:351–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

