Synthesis and Structure-Activity Relationships of Benzothienothiazepinone Inhibitors of Protein Kinase D
- PMID: 21617763
- PMCID: PMC3100199
- DOI: 10.1021/ml100230n
Synthesis and Structure-Activity Relationships of Benzothienothiazepinone Inhibitors of Protein Kinase D
Abstract
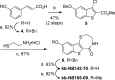
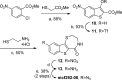
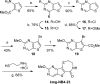
Protein kinase D (PKD) is a member of a novel family of serine/threonine kinases that regulate fundamental cellular processes. PKD is implicated in the pathogenesis of several diseases, including cancer. Progress in understanding the biological functions and therapeutic potential of PKD has been hampered by the lack of specific inhibitors. The benzoxoloazepinolone CID755673 was recently identified as the first potent and selective PKD inhibitor. The study of structure-activity relationships (SAR) of this lead structure led to further improvements in PKD1 potency. We describe herein the synthesis and biological evaluation of novel benzothienothiazepinone analogs. We achieved a ten-fold increase in the in vitro PKD1 inhibitory potency for the second generation lead kb-NB142-70 and accomplished a transition to an almost equally potent novel pyrimidine scaffold, while maintaining excellent target selectivity. These promising results will guide the design of pharmacological tools to dissect PKD function and pave the way for the development of potential anti-cancer agents.
Figures
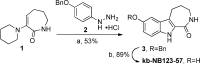
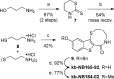
References
-
- Newton A. C. Protein Kinase C: Structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem. Rev. 2001, 101, 2353–2364. - PubMed
-
- Yang C.; Kazanietz M. G. Divergence and complexities in DAG signaling: looking beyond PKC. Trends Pharmacol. Sci. 2003, 24, 602–608. - PubMed
-
- Rozengurt E.; Rey O.; Waldron R. T. Protein kinase D signaling. J. Biol. Chem. 2005, 280, 13205–13208. - PubMed
-
- Sinnett-Smith J.; Jacamo R.; Kui R.; Wang Y. M.; Young S. H.; Rey O.; Waldron R. T.; Rozengurt E. Protein kinase D mediates mitogenic signaling by Gq-coupled receptors through protein kinase C-independent regulation of activation loop Ser744 and Ser748 phosphorylation. J. Biol. Chem. 2009, 284, 13434–13445. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

