Up-regulation of thromboxane A₂ impairs cerebrovascular eNOS function in aging atherosclerotic mice
- PMID: 21617900
- PMCID: PMC3682985
- DOI: 10.1007/s00424-011-0973-y
Up-regulation of thromboxane A₂ impairs cerebrovascular eNOS function in aging atherosclerotic mice
Abstract
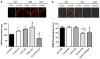
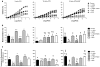
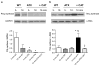
We previously reported that in healthy mouse cerebral arteries, endothelial nitric oxide synthase (eNOS) produces H₂O₂, leading to endothelium-dependent dilation. In contrast, thromboxane A₂ (TXA₂), a potent pro-oxidant and pro-inflammatory endogenous vasoconstrictor, is associated with eNOS dysfunction. Our objectives were to elucidate whether (1) the cerebrovascular eNOS-H₂O₂ pathway was sensitive to oxidative stress associated with aging and dyslipidemia and (2) TXA₂ contributed to cerebral eNOS dysfunction. Atherosclerotic (ATX = LDLR(-/-); hApoB(+/+)) and wild-type (WT) control mice were used at 3 and 12 months old (m/o). Three-m/o ATX mice were treated with the cardio-protective polyphenol catechin for 9 months. Dilations to ACh and the simultaneous eNOS-derived H₂O₂ production were recorded in isolated pressurized cerebral arteries. The age-associated decrease in cerebral eNOS-H₂O₂ pathway observed in WT was premature in ATX mice, decreasing at 3 m/o and abolished at 12 m/o. Thromboxane synthase inhibition by furegrelate increased dilations at 12 months in WT and at 3 and 12 months in ATX mice, suggesting an anti-dilatory role of TXA₂ with age hastened by dyslipidemia. In addition, the non-selective NADP(H) oxidase inhibitor apocynin improved the eNOS-H₂O₂ pathway only in 12-m/o ATX mice. Catechin normalized the function of this pathway, which became sensitive to L-NNA and insensitive to furegrelate or apocynin; catechin also prevented the rise in TXA₂ synthase expression. In conclusion, the age-dependent cerebral endothelial dysfunction is precocious in dyslipidemia and involves TXA₂ production that limits eNOS activity. Preventive catechin treatment reduced the impact of endogenous TXA₂ on the control of cerebral tone and maintained eNOS function.
Conflict of interest statement
Figures




Similar articles
-
Catechin prevents severe dyslipidemia-associated changes in wall biomechanics of cerebral arteries in LDLr-/-:hApoB+/+ mice and improves cerebral blood flow.Am J Physiol Heart Circ Physiol. 2012 Mar 15;302(6):H1330-9. doi: 10.1152/ajpheart.01044.2011. Epub 2012 Jan 20. Am J Physiol Heart Circ Physiol. 2012. PMID: 22268108 Free PMC article.
-
A change in the redox environment and thromboxane A2 production precede endothelial dysfunction in mice.Am J Physiol Heart Circ Physiol. 2007 Oct;293(4):H2508-15. doi: 10.1152/ajpheart.00352.2007. Epub 2007 Jul 20. Am J Physiol Heart Circ Physiol. 2007. PMID: 17644574
-
Catechin treatment improves cerebrovascular flow-mediated dilation and learning abilities in atherosclerotic mice.Am J Physiol Heart Circ Physiol. 2011 Mar;300(3):H1032-43. doi: 10.1152/ajpheart.00410.2010. Epub 2010 Dec 24. Am J Physiol Heart Circ Physiol. 2011. PMID: 21186270 Free PMC article.
-
Endothelial nitric oxide synthase activation leads to dilatory H2O2 production in mouse cerebral arteries.Cardiovasc Res. 2007 Jan 1;73(1):73-81. doi: 10.1016/j.cardiores.2006.10.005. Epub 2006 Oct 13. Cardiovasc Res. 2007. PMID: 17113574
-
Subcellular localization of oxidants and redox modulation of endothelial nitric oxide synthase.Circ J. 2012;76(11):2497-512. doi: 10.1253/circj.cj-12-1207. Epub 2012 Oct 18. Circ J. 2012. PMID: 23075817 Review.
Cited by
-
Lack of angiopoietin-like-2 expression limits the metabolic stress induced by a high-fat diet and maintains endothelial function in mice.J Am Heart Assoc. 2014 Aug 15;3(4):e001024. doi: 10.1161/JAHA.114.001024. J Am Heart Assoc. 2014. PMID: 25128474 Free PMC article.
-
Catechin prevents severe dyslipidemia-associated changes in wall biomechanics of cerebral arteries in LDLr-/-:hApoB+/+ mice and improves cerebral blood flow.Am J Physiol Heart Circ Physiol. 2012 Mar 15;302(6):H1330-9. doi: 10.1152/ajpheart.01044.2011. Epub 2012 Jan 20. Am J Physiol Heart Circ Physiol. 2012. PMID: 22268108 Free PMC article.
-
Effects of BM-573 on Endothelial Dependent Relaxation and Increased Blood Pressure at Early Stages of Atherosclerosis.PLoS One. 2016 Mar 28;11(3):e0152579. doi: 10.1371/journal.pone.0152579. eCollection 2016. PLoS One. 2016. PMID: 27019366 Free PMC article.
-
The senolytic ABT-263 improves cognitive functions in middle-aged male, but not female, atherosclerotic LDLr-/-;hApoB100+/+ mice.Geroscience. 2025 Jun;47(3):4577-4600. doi: 10.1007/s11357-025-01563-3. Epub 2025 Feb 21. Geroscience. 2025. PMID: 39982668 Free PMC article.
-
The Role of Obesity-Induced Perivascular Adipose Tissue (PVAT) Dysfunction in Vascular Homeostasis.Nutrients. 2021 Oct 28;13(11):3843. doi: 10.3390/nu13113843. Nutrients. 2021. PMID: 34836100 Free PMC article. Review.
References
-
- Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. - PubMed
-
- Ansar S, Larsen C, Maddahi A, Edvinsson L. Subarachnoid hemorrhage induces enhanced expression of thromboxane A2 receptors in rat cerebral arteries. Brain Res. 2010;1316:163–172. - PubMed
-
- Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. - PubMed
-
- Crawford RS, Kirk EA, Rosenfeld ME, LeBoeuf RC, Chait A. Dietary antioxidants inhibit development of fatty streak lesions in the LDL receptor-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:1506–1513. - PubMed
-
- Didion SP, Heistad DD, Faraci FM. Mechanisms that produce nitric oxide-mediated relaxation of cerebral arteries during atherosclerosis. Stroke. 2001;32:761–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

