Genetic tracing of Nav1.8-expressing vagal afferents in the mouse
- PMID: 21618224
- PMCID: PMC3306808
- DOI: 10.1002/cne.22667
Genetic tracing of Nav1.8-expressing vagal afferents in the mouse
Abstract
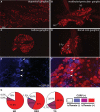
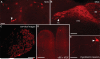
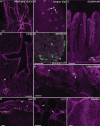
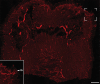
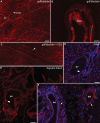
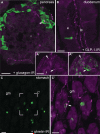
Nav1.8 is a tetrodotoxin-resistant sodium channel present in large subsets of peripheral sensory neurons, including both spinal and vagal afferents. In spinal afferents, Nav1.8 plays a key role in signaling different types of pain. Little is known, however, about the exact identity and role of Nav1.8-expressing vagal neurons. Here we generated mice with restricted expression of tdTomato fluorescent protein in all Nav1.8-expressing afferent neurons. As a result, intense fluorescence was visible in the cell bodies, central relays, and sensory endings of these neurons, revealing the full extent of their innervation sites in thoracic and abdominal viscera. For instance, vagal and spinal Nav1.8-expressing endings were seen clearly within the gastrointestinal mucosa and myenteric plexus, respectively. In the gastrointestinal muscle wall, labeled endings included a small subset of vagal tension receptors but not any stretch receptors. We also examined the detailed innervation of key metabolic tissues such as liver and pancreas and evaluated the anatomical relationship of Nav1.8-expressing vagal afferents with select enteroendocrine cells (i.e., ghrelin, glucagon, GLP-1). Specifically, our data revealed the presence of Nav1.8-expressing vagal afferents in several metabolic tissues and varying degrees of proximity between Nav1.8-expressing mucosal afferents and enteroendocrine cells, including apparent neuroendocrine apposition. In summary, this study demonstrates the power and versatility of the Cre-LoxP technology to trace identified visceral afferents, and our data suggest a previously unrecognized role for Nav1.8-expressing vagal neurons in gastrointestinal functions.
Copyright © 2011 Wiley-Liss, Inc.
Figures


References
-
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. - PubMed
-
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
-
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. - PubMed
-
- Altschuler SM, Bao XM, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283:248–268. - PubMed
-
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

