15N solid-state NMR as a probe of flavin H-bonding
- PMID: 21619002
- PMCID: PMC3156030
- DOI: 10.1021/jp202138d
15N solid-state NMR as a probe of flavin H-bonding
Abstract
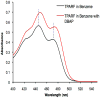
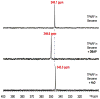
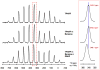
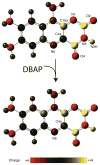
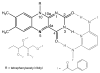
Flavins mediate a wide variety of chemical reactions in biology. To learn how one cofactor can be made to execute different reactions in different enzymes, we are developing solid-state NMR (SSNMR) to probe the flavin electronic structure, via the (15)N chemical shift tensor principal values (δ(ii)). We find that SSNMR has superior responsiveness to H-bonds, compared to solution NMR. H-bonding to a model of the flavodoxin active site produced an increase of 10 ppm in the δ(11) of N5, although none of the H-bonds directly engage N5, and solution NMR detected only a 4 ppm increase in the isotropic chemical shift (δ(iso)). Moreover SSNMR responded differently to different H-bonding environments, as H-bonding with water caused δ(11) to decrease by 6 ppm, whereas δ(iso) increased by less than 1 ppm. Our density functional theoretical (DFT) calculations reproduce the observations, validating the use of computed electronic structures to understand how H-bonds modulate the flavin's reactivity.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

