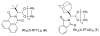Catalytic asymmetric C-H insertions of rhodium(II) azavinyl carbenes
- PMID: 21619004
- PMCID: PMC3142877
- DOI: 10.1021/ja202969z
Catalytic asymmetric C-H insertions of rhodium(II) azavinyl carbenes
Abstract
A highly efficient enantioselective C-H insertion of azavinyl carbenes into unactivated alkanes has been developed. These transition metal carbenes are directly generated from readily available and stable 1-sulfonyl-1,2,3-triazoles in the presence of chiral Rh(II) carboxylates and are used for C-H functionalization of alkanes to access a variety of β-chiral sulfonamides.
Figures
References
-
-
For reviews, see: Davies HML, Morton D. Chem Soc Rev. 2011;40:1857.Doyle MP, Duffy R, Ratnikov M, Zhou L. Chem Rev. 2010;110:704.Davies HML, Manning JR. Nature. 2008;451:417.Davies HML, Beckwith REJ. Chem Rev. 2003;103:2861.Lebel H, Marcoux JF, Molinaro C, Charette AB. Chem Rev. 2003;103:977.Doyle MP, McKervey MA, Ye T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds: From Cyclopropanes to Ylides. Wiley; NY: 1998.
-
-
- Davies HML, Hansen T. J Am Chem Soc. 1997;119:9075.
- Davies HML, Hansen T, Churchill MR. J Am Chem Soc. 2000;122:3063.
-
-
Horneff T, Chuprakov S, Chernyak N, Gevorgyan V, Fokin VV. J Am Chem Soc. 2008;130:14972.Chuprakov S, Kwok SW, Zhang L, Lercher L, Fokin VV. J Am Chem Soc. 2009;131:18034.Grimster N, Zhang L, Fokin VV. J Am Chem Soc. 2010;132:2510.For related 2-pyrydyl analogues, see: Davies HML, Townsend RJ. J Org Chem. 2001;66:6595.Chuprakov S, Gevorgyan V. Org Lett. 2007;9:4463.Chuprakov S, Hwang FW, Gevorgyan V. Angew Chem, Int Ed. 2007;46:4757.
-
-
-
For recent applications, see: Müller P, Allenbach YF, Robert E. Tetrahedron: Asymmetry. 2003;14:779.Müller P, Bernardinelli G, Allenbach YF, Ferry M, Flack HD. Org Lett. 2004;6:1725.Marcoux D, Charette AB. Angew Chem, Int Ed. 2008;47:10155.Marcoux D, Azzi S, Charette AB. J Am Chem Soc. 2009;131:6970.DeAngelis A, Shurtleff VW, Dmitrenko O, Fox JM. J Am Chem Soc. 2011;133:1650.See also ref. 3b–c.
-
-
-
Similarly to the previously reported Rh-catalyzed C–H insertion with diazoesters, dry and degassed solvents were used to ensure efficiency of the reaction. See Supporting Information for details. See also ref. 2b.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources