Real-time visualization of heterotrimeric G protein Gq activation in living cells
- PMID: 21619590
- PMCID: PMC3129320
- DOI: 10.1186/1741-7007-9-32
Real-time visualization of heterotrimeric G protein Gq activation in living cells
Abstract
Background: Gq is a heterotrimeric G protein that plays an important role in numerous physiological processes. To delineate the molecular mechanisms and kinetics of signalling through this protein, its activation should be measurable in single living cells. Recently, fluorescence resonance energy transfer (FRET) sensors have been developed for this purpose.
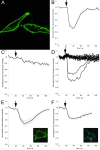
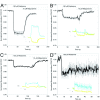
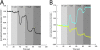
Results: In this paper, we describe the development of an improved FRET-based Gq activity sensor that consists of a yellow fluorescent protein (YFP)-tagged Gγ2 subunit and a Gαq subunit with an inserted monomeric Turquoise (mTurquoise), the best cyan fluorescent protein variant currently available. This sensor enabled us to determine, for the first time, the kon (2/s) of Gq activation. In addition, we found that the guanine nucleotide exchange factor p63RhoGEF has a profound effect on the number of Gq proteins that become active upon stimulation of endogenous histamine H1 receptors. The sensor was also used to measure ligand-independent activation of the histamine H1 receptor (H1R) upon addition of a hypotonic stimulus.
Conclusions: Our observations reveal that the application of a truncated mTurquoise as donor and a YFP-tagged Gγ2 as acceptor in FRET-based Gq activity sensors substantially improves their dynamic range. This optimization enables the real-time single cell quantification of Gq signalling dynamics, the influence of accessory proteins and allows future drug screening applications by virtue of its sensitivity.
Figures

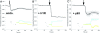

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

