Transcriptional dysregulation of 5-HT1A autoreceptors in mental illness
- PMID: 21619616
- PMCID: PMC3130656
- DOI: 10.1186/1756-6606-4-21
Transcriptional dysregulation of 5-HT1A autoreceptors in mental illness
Abstract
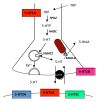
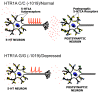
The serotonin-1A (5-HT1A) receptor is among the most abundant and widely distributed 5-HT receptors in the brain, but is also expressed on serotonin neurons as an autoreceptor where it plays a critical role in regulating the activity of the entire serotonin system. Over-expression of the 5-HT1A autoreceptor has been implicated in reducing serotonergic neurotransmission, and is associated with major depression and suicide. Extensive characterization of the transcriptional regulation of the 5-HT1A gene (HTR1A) using cell culture systems has revealed a GC-rich "housekeeping" promoter that non-selectively drives its expression; this is flanked by a series of upstream repressor elements for REST, Freud-1/CC2D1A and Freud-2/CC2D1B factors that not only restrict its expression to neurons, but may also regulate the level of expression of 5-HT1A receptors in various subsets of neurons, including serotonergic neurons. A separate set of allele-specific factors, including Deaf1, Hes1 and Hes5 repress at the HTR1A C(-1019)G (rs6295) polymorphism in serotonergic neurons in culture, as well as in vivo. Pet1, an obligatory enhancer for serotonergic differentiation, has been identified as a potent activator of 5-HT1A autoreceptor expression. Taken together, these results highlight an integrated regulation of 5-HT1A autoreceptors that differs in several aspects from regulation of post-synaptic 5-HT1A receptors, and could be selectively targeted to enhance serotonergic neurotransmission.
Figures
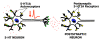
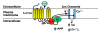
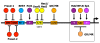
References
-
- WHO. The World Health Report 2001. Mental Health, New Understanding, New Hope. Geneva, Switzerland: WHO Marketing and Dissemination; 2001.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

