Vaccination coverage and timeliness in three South African areas: a prospective study
- PMID: 21619642
- PMCID: PMC3126743
- DOI: 10.1186/1471-2458-11-404
Vaccination coverage and timeliness in three South African areas: a prospective study
Abstract
Background: Timely vaccination is important to induce adequate protective immunity. We measured vaccination timeliness and vaccination coverage in three geographical areas in South Africa.
Methods: This study used vaccination information from a community-based cluster-randomized trial promoting exclusive breastfeeding in three South African sites (Paarl in the Western Cape Province, and Umlazi and Rietvlei in KwaZulu-Natal) between 2006 and 2008. Five interview visits were carried out between birth and up to 2 years of age (median follow-up time 18 months), and 1137 children were included in the analysis. We used Kaplan-Meier time-to-event analysis to describe vaccination coverage and timeliness in line with the Expanded Program on Immunization for the first eight vaccines. This included Bacillus Calmette-Guérin (BCG), four oral polio vaccines and 3 doses of the pentavalent vaccine which protects against diphtheria, pertussis, tetanus, hepatitis B and Haemophilus influenzae type B.
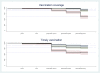
Results: The proportion receiving all these eight recommended vaccines were 94% in Paarl (95% confidence interval [CI] 91-96), 62% in Rietvlei (95%CI 54-68) and 88% in Umlazi (95%CI 84-91). Slightly fewer children received all vaccines within the recommended time periods. The situation was worst for the last pentavalent- and oral polio vaccines. The hazard ratio for incomplete vaccination was 7.2 (95%CI 4.7-11) for Rietvlei compared to Paarl.
Conclusions: There were large differences between the different South African sites in terms of vaccination coverage and timeliness, with the poorer areas of Rietvlei performing worse than the better-off areas in Paarl. The vaccination coverage was lower for the vaccines given at an older age. There is a need for continued efforts to improve vaccination coverage and timeliness, in particular in rural areas.
Trial registration number: ClinicalTrials.gov: NCT00397150.
Figures

References
-
- The measles epidemic. The problems, barriers, and recommendations. The National Vaccine Advisory Committee. JAMA. 1991;266(11):1547–1552. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

