Practical performance evaluation of a 10k × 10k CCD for electron cryo-microscopy
- PMID: 21619932
- PMCID: PMC3150461
- DOI: 10.1016/j.jsb.2011.05.012
Practical performance evaluation of a 10k × 10k CCD for electron cryo-microscopy
Abstract
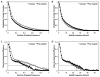
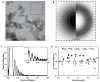
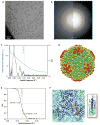
Electron cryo-microscopy (cryo-EM) images are commonly collected using either charge-coupled devices (CCD) or photographic film. Both film and the current generation of 16 megapixel (4k × 4k) CCD cameras have yielded high-resolution structures. Yet, despite the many advantages of CCD cameras, more than two times as many structures of biological macromolecules have been published in recent years using photographic film. The continued preference to film, especially for subnanometer-resolution structures, may be partially influenced by the finer sampling and larger effective specimen imaging area offered by film. Large format digital cameras may finally allow them to overtake film as the preferred detector for cryo-EM. We have evaluated a 111-megapixel (10k × 10k) CCD camera with a 9 μm pixel size. The spectral signal-to-noise ratios of low dose images of carbon film indicate that this detector is capable of providing signal up to at least 2/5 Nyquist frequency potentially retrievable for 3D reconstructions of biological specimens, resulting in more than double the effective specimen imaging area of existing 4k × 4k CCD cameras. We verified our estimates using frozen-hydrated ε15 bacteriophage as a biological test specimen with previously determined structure, yielding a ∼7 Å resolution single particle reconstruction from only 80 CCD frames. Finally, we explored the limits of current CCD technology by comparing the performance of this detector to various CCD cameras used for recording data yielding subnanometer resolution cryo-EM structures submitted to the electron microscopy data bank (http://www.emdatabank.org/).
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
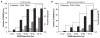



References
-
- Booth CR, Jiang W, Baker ML, Zhou ZH, Ludtke SJ, et al. A 9 Å single particle reconstruction from CCD captured images on a 200 kV electron cryo-microscope. J Struct Biol. 2004;147:116–127. - PubMed
-
- Booth CR, Jakana J, Chiu W. Assessing the capabilities of a 4k×4k CCD camera for electron cryo-microscopy. J Struct Biol. 2006;156:556–63. - PubMed
-
- Brink J, Chiu W. Applications of a slow-scan CCD camera in protein electron crystallography. J Struct Biol. 1994;113:23–34. - PubMed
-
- Carragher B, Kisseberth N, Kriegman D, Milligan RA, Potter CS, et al. Leginon: an automated system for acquisition of images from vitreous ice specimens. J Struct Biol. 2000;132:33–45. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

