Pharmacokinetics of 17-hydroxyprogesterone caproate in multifetal gestation
- PMID: 21620357
- PMCID: PMC3165062
- DOI: 10.1016/j.ajog.2011.03.028
Pharmacokinetics of 17-hydroxyprogesterone caproate in multifetal gestation
Abstract
Objective: The purpose of this study was to define the pharmacokinetic parameters of 17-hydroxyprogesterone caproate (17-OHPC) in multifetal gestation.
Study design: Blood was obtained at 24-28 weeks' gestation and at 32-35 weeks gestation in 97 women with twin and 26 women with triplet gestation who were receiving 17-OHPC. Six of the women with twins had daily blood sampling for 7 days between 24 and 28 weeks' gestation, and pharmacokinetic parameters were estimated with the use of noncompartmental analysis. Modeling was applied to estimate the population parameters and to simulate various treatment scenarios.
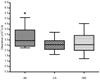
Results: The apparent half-life of 17-OHPC was 10 days. Body mass index significantly impacted 17-OHPC concentrations, but fetal number and parity did not. Apparent clearance was significantly greater in African American than in white women (P = .025).
Conclusion: This is the first pharmacokinetic analysis of 17-OHPC in pregnant women. Determination of half-life, covariates that affect plasma 17-OHPC concentrations, and the modeling of drug behavior provide insights into this drug's pharmacologic properties during multifetal pregnancy.
Trial registration: ClinicalTrials.gov NCT00099164.
Copyright © 2011 Mosby, Inc. All rights reserved.
Figures
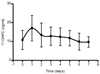


effect of BMI (18, 45 and 27) on plasma concentration time profiles,
effect of adding a loading dose of 1000 mg to the currently recommended regimen, and
effect of changing the dosing schedule from 250 mg once weekly to 500 mg every 2 weeks.
References
-
- Meis, et al. for the NICHD Maternal-Fetal Medicine Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385. - PubMed
-
- Rouse DJ, et al. for the NICHD Maternal-Fetal Medicine Units Network. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N. Engl J Med. 2007;357:454–461. - PubMed
-
- Combs CA, Carite T, Maurel K, Das A, Porto M. Obstetrix Collaborative Network. Failure of 17-hydroxyprogesterone to reduce neonatal morbidity or prolong triplet pregnancy: a double-blind, randomized clinical trial. Am J Obstet Gynecol. 2010 Sep;203(3):248.e1–248.e9. - PubMed
-
- Berghella V, Figueroa D, Szychowski JM, Owen J, Hankins GD, Iams JD, Sheffield JS, Perez-Delboy A, Wing DA, Guzman ER. Vaginal Ultrasound Trial Consortium. 17-alpha-hydroxyprogesterone caproate for the prevention of preterm birth in women with prior preterm birth and a short cervical length. Am J Obstet Gynecol. 2010 Apr;202(4):351.e1–351.e6. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HD36801/HD/NICHD NIH HHS/United States
- UG1 HD027869/HD/NICHD NIH HHS/United States
- U10 HD034136/HD/NICHD NIH HHS/United States
- U10 HD040485/HD/NICHD NIH HHS/United States
- HD40485/HD/NICHD NIH HHS/United States
- HD40560/HD/NICHD NIH HHS/United States
- U10 HD040560/HD/NICHD NIH HHS/United States
- U10 HD040512/HD/NICHD NIH HHS/United States
- U01 HD036801/HD/NICHD NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- U10 HD040500/HD/NICHD NIH HHS/United States
- U10 HD040544/HD/NICHD NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- UG1 HD034116/HD/NICHD NIH HHS/United States
- HD27869/HD/NICHD NIH HHS/United States
- HD34136/HD/NICHD NIH HHS/United States
- UG1 HD040560/HD/NICHD NIH HHS/United States
- HD27860/HD/NICHD NIH HHS/United States
- UG1 HD027915/HD/NICHD NIH HHS/United States
- HD40512/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
- UG1 HD040512/HD/NICHD NIH HHS/United States
- HD40545/HD/NICHD NIH HHS/United States
- U10 HD034116/HD/NICHD NIH HHS/United States
- HD21410/HD/NICHD NIH HHS/United States
- U10 HD027869/HD/NICHD NIH HHS/United States
- U10 HD027917/HD/NICHD NIH HHS/United States
- HD34116/HD/NICHD NIH HHS/United States
- U10 HD027915/HD/NICHD NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- UG1 HD040485/HD/NICHD NIH HHS/United States
- U10 HD027860/HD/NICHD NIH HHS/United States
- HD40500/HD/NICHD NIH HHS/United States
- U10 HD034208/HD/NICHD NIH HHS/United States
- HD34208/HD/NICHD NIH HHS/United States
- HD27915/HD/NICHD NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- HD27917/HD/NICHD NIH HHS/United States
- U10 HD021410/HD/NICHD NIH HHS/United States
- U10 HD036801/HD/NICHD NIH HHS/United States
- U24 HD036801/HD/NICHD NIH HHS/United States
- U10 HD040545/HD/NICHD NIH HHS/United States
- HD40544/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

