Acute retinal arterial occlusive disorders
- PMID: 21620994
- PMCID: PMC3137709
- DOI: 10.1016/j.preteyeres.2011.05.001
Acute retinal arterial occlusive disorders
Abstract
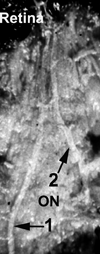
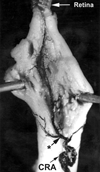
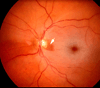
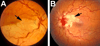
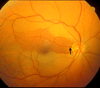
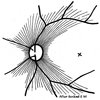
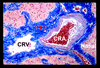
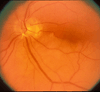
The initial section deals with basic sciences; among the various topics briefly discussed are the anatomical features of ophthalmic, central retinal and cilioretinal arteries which may play a role in acute retinal arterial ischemic disorders. Crucial information required in the management of central retinal artery occlusion (CRAO) is the length of time the retina can survive following that. An experimental study shows that CRAO for 97min produces no detectable permanent retinal damage but there is a progressive ischemic damage thereafter, and by 4h the retina has suffered irreversible damage. In the clinical section, I discuss at length various controversies on acute retinal arterial ischemic disorders. Classification of acute retinal arterial ischemic disorders: These are of 4 types: CRAO, branch retinal artery occlusion (BRAO), cotton wool spots and amaurosis fugax. Both CRAO and BRAO further comprise multiple clinical entities. Contrary to the universal belief, pathogenetically, clinically and for management, CRAO is not one clinical entity but 4 distinct clinical entities - non-arteritic CRAO, non-arteritic CRAO with cilioretinal artery sparing, arteritic CRAO associated with giant cell arteritis (GCA) and transient non-arteritic CRAO. Similarly, BRAO comprises permanent BRAO, transient BRAO and cilioretinal artery occlusion (CLRAO), and the latter further consists of 3 distinct clinical entities - non-arteritic CLRAO alone, non-arteritic CLRAO associated with central retinal vein occlusion and arteritic CLRAO associated with GCA. Understanding these classifications is essential to comprehend fully various aspects of these disorders. Central retinal artery occlusion: The pathogeneses, clinical features and management of the various types of CRAO are discussed in detail. Contrary to the prevalent belief, spontaneous improvement in both visual acuity and visual fields does occur, mainly during the first 7 days. The incidence of spontaneous visual acuity improvement during the first 7 days differs significantly (p<0.001) among the 4 types of CRAO; among them, in eyes with initial visual acuity of counting finger or worse, visual acuity improved, remained stable or deteriorated in non-arteritic CRAO in 22%, 66% and 12% respectively; in non-arteritic CRAO with cilioretinal artery sparing in 67%, 33% and none respectively; and in transient non-arteritic CRAO in 82%, 18% and none respectively. Arteritic CRAO shows no change. Recent studies have shown that administration of local intra-arterial thrombolytic agent not only has no beneficial effect but also can be harmful. Prevalent multiple misconceptions on CRAO are discussed. Branch retinal artery occlusion: Pathogeneses, clinical features and management of various types of BRAO are discussed at length. The natural history of visual acuity outcome shows a final visual acuity of 20/40 or better in 89% of permanent BRAO cases, 100% of transient BRAO and 100% of non-arteritic CLRAO alone. Cotton wools spots: These are common, non-specific acute focal retinal ischemic lesions, seen in many retinopathies. Their pathogenesis and clinical features are discussed in detail. Amaurosis fugax: Its pathogenesis, clinical features and management are described.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The author has no conflict of interest.
Figures

















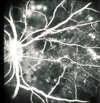
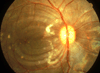
References
-
- Ahuja RM, Chaturvedi S, Eliott D, Joshi N, Puklin JE, Abrams GW. Mechanisms of retinal arterial occlusive disease in African American and Caucasian patients. Stroke. 1999;30:1506–1509. - PubMed
-
- Anderson DC, Kappelle LJ, Eliasziw M, Babikian VL, Pearce LA, Barnett HJ. Occurrence of hemispheric and retinal ischemia in atrial fibrillation compared with carotid stenosis. Stroke. 2002;33:1963–1967. - PubMed
-
- Anderson DR. Glaucoma, capillaries and pericytes. 1. Blood flow regulation. Ophthalmologica. 1996;210:257–262. - PubMed
-
- Anderson DR, Davis EB. Glaucoma, capillaries and pericytes. 2. Identification and characterization of retinal pericytes in culture. Ophthalmologica. 1996;210:263–268. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

