Spatiotemporal delivery of bone morphogenetic protein enhances functional repair of segmental bone defects
- PMID: 21621027
- PMCID: PMC3143222
- DOI: 10.1016/j.bone.2011.05.010
Spatiotemporal delivery of bone morphogenetic protein enhances functional repair of segmental bone defects
Abstract
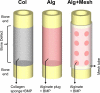
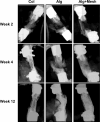
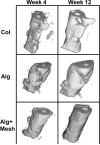
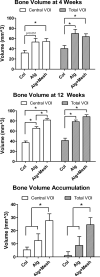
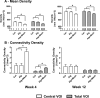
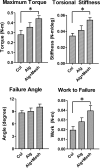
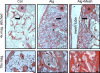
Osteogenic growth factors that promote endogenous repair mechanisms hold considerable potential for repairing challenging bone defects. The local delivery of one such growth factor, bone morphogenetic protein (BMP), has been successfully translated to clinical practice for spinal fusion and bone fractures. However, improvements are needed in the spatial and temporal control of BMP delivery to avoid the currently used supraphysiologic doses and the concomitant adverse effects. We have recently introduced a hybrid protein delivery system comprised of two parts: a perforated nanofibrous mesh that spatially confines the defect region and a functionalized alginate hydrogel that provides temporal growth factor release kinetics. Using this unique spatiotemporal delivery system, we previously demonstrated BMP-mediated functional restoration of challenging 8mm femoral defects in a rat model. In this study, we compared the efficacy of the hybrid system in repairing segmental bone defects to that of the current clinical standard, collagen sponge, at the same dose of recombinant human BMP-2. In addition, we investigated the specific role of the nanofibrous mesh tube on bone regeneration. Our results indicate that the hybrid delivery system significantly increased bone regeneration and improved biomechanical function compared to collagen sponge delivery. Furthermore, we observed that presence of the nanofiber mesh tube was essential to promote maximal mineralized matrix synthesis, prevent extra-anatomical mineralization, and guide an integrated pattern of bone formation. Together, these results suggest that spatiotemporal strategies for osteogenic protein delivery may enhance clinical outcomes by improving localized protein retention.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
References
-
- Cypher TJ, Grossman JP. Biological principles of bone graft healing. J Foot Ankle Surg. 1996;35:413–7. - PubMed
-
- Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–9. - PubMed
-
- Berrey BH, Jr., Lord CF, Gebhardt MC, Mankin HJ. Fractures of allografts. Frequency, treatment, and end-results. J Bone Joint Surg Am. 1990;72:825–33. - PubMed
-
- Wheeler DL, Enneking WF. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res. 2005;435:36–42. - PubMed
-
- Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

