Oxygen supply from the bird's eye perspective: globin E is a respiratory protein in the chicken retina
- PMID: 21622558
- PMCID: PMC3143615
- DOI: 10.1074/jbc.M111.224634
Oxygen supply from the bird's eye perspective: globin E is a respiratory protein in the chicken retina
Abstract
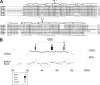
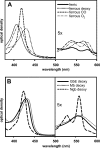
The visual process in the vertebrate eye requires high amounts of metabolic energy and thus oxygen. Oxygen supply of the avian retina is a challenging task because birds have large eyes, thick retinae, and high metabolic rates but neither deep retinal nor superficial capillaries. Respiratory proteins such as myoglobin may enhance oxygen supply to certain tissues, and thus the mammalian retina harbors high amounts of neuroglobin. Globin E (GbE) was recently identified as an eye-specific globin of chicken (Gallus gallus). Orthologous GbE genes were found in zebra finch and turkey genomes but appear to be absent in non-avian vertebrate classes. Analyses of globin phylogeny and gene synteny showed an ancient origin of GbE but did not help to assign it to any specific globin type. We show that the photoreceptor cells of the chicken retina have a high level of GbE protein, which accumulates to ∼10 μM in the total eye. Quantitative real-time RT-PCR revealed an ∼50,000-fold higher level of GbE mRNA in the eye than in the brain. Spectroscopic analysis and ligand binding kinetics of recombinant chicken GbE reveal a penta-coordinated globin with an oxygen affinity of P(50) = 5.8 torrs at 25 °C and 15 torrs at 41 °C. Together these data suggest that GbE helps to sustain oxygen supply to the avian retina.
Figures
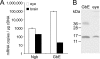


References
-
- Dickerson A. E., Greis I. (1983) Hemoglobin: Structure, Function, Evolution, and Pathology, Benjamin-Cummings Publishing Company, Inc., Menlo Park, CA
-
- Wittenberg J. B., Wittenberg B. A. (2003) J. Exp. Biol. 206, 2011–2020 - PubMed
-
- Burmester T., Weich B., Reinhardt S., Hankeln T. (2000) Nature 407, 520–523 - PubMed
-
- Burmester T., Hankeln T. (2009) J. Exp. Biol. 212, 1423–1428 - PubMed
-
- Hankeln T., Ebner B., Fuchs C., Gerlach F., Haberkamp M., Laufs T. L., Roesner A., Schmidt M., Weich B., Wystub S., Saaler-Reinhardt S., Reuss S., Bolognesi M., De Sanctis D., Marden M. C., Kiger L., Moens L., Dewilde S., Nevo E., Avivi A., Weber R. E., Fago A., Burmester T. (2005) J. Inorg. Biochem. 99, 110–119 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

