Sorafenib enhances pemetrexed cytotoxicity through an autophagy-dependent mechanism in cancer cells
- PMID: 21622715
- PMCID: PMC3139015
- DOI: 10.1158/0008-5472.CAN-11-0898
Sorafenib enhances pemetrexed cytotoxicity through an autophagy-dependent mechanism in cancer cells
Abstract
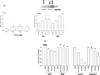
Pemetrexed (ALIMTA, Lilly) is a folate antimetabolite that has been approved by the U.S. Food and Drug Administration for the treatment of non-small cell lung cancer and has been shown to stimulate autophagy. In the present study, we sought to further understand the role of autophagy in response to pemetrexed and to test if combination therapy could enhance the level of toxicity through altered autophagy in tumor cells. The multikinase inhibitor sorafenib (Nexavar, Bayer), used in the treatment of renal and hepatocellular carcinoma, suppresses tumor angiogenesis and promotes autophagy in tumor cells. We found that sorafenib interacted in a greater than additive fashion with pemetrexed to increase autophagy and to kill a diverse array of tumor cell types. Tumor cell types that displayed high levels of cell killing after combination treatment showed elevated levels of AKT, p70 S6K, and/or phosphorylated mTOR, in addition to class III receptor tyrosine kinases such as platelet-derived growth factor receptor beta and VEGF receptors, known in vivo targets of sorafenib. In xenograft and in syngeneic animal models of mammary carcinoma and glioblastoma, the combination of sorafenib and pemetrexed suppressed tumor growth without deleterious effects on normal tissues or animal body mass. Taken together, the data suggest that premexetred and sorafenib act synergistically to enhance tumor killing via the promotion of a toxic form of autophagy that leads to activation of the intrinsic apoptosis pathway, and predict that combination treatment represents a future therapeutic option in the treatment of solid tumors.
©2011 AACR.
Figures









References
-
- Jarmuła A. Antifolate Inhibitors of Thymidylate Synthase as Anticancer Drugs. Mini Rev Med Chem. 2010 Sep 21; [Epub ahead of print] - PubMed
-
- Fleeman N, Bagust A, McLeod C, Greenhalgh J, Boland A, Dundar Y, Dickson R, Tudur Smith C, Davis H, Green J, Pearson M. Pemetrexed for the first-line treatment of locally advanced or metastatic non-small cell lung cancer. Health Technol Assess. 2010;14 S1:47–53. - PubMed
-
- Chattopadhyay S, Zhao R, Krupenko SA, Krupenko N, Goldman ID. The inverse relationship between reduced folate carrier function and pemetrexed activity in a human colon cancer cell line. Mol Cancer Ther. 2006;5:438–449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA039687/CA/NCI NIH HHS/United States
- R01 CA085289/CA/NCI NIH HHS/United States
- R01 CA108520/CA/NCI NIH HHS/United States
- R01-CA77141/CA/NCI NIH HHS/United States
- R01-CA150214/CA/NCI NIH HHS/United States
- P01-CA104177/CA/NCI NIH HHS/United States
- R01-CA141703/CA/NCI NIH HHS/United States
- R01-CA63753/CA/NCI NIH HHS/United States
- R01 CA063753/CA/NCI NIH HHS/United States
- U54CA113001/CA/NCI NIH HHS/United States
- R01 CA141703/CA/NCI NIH HHS/United States
- R01 DK052825/DK/NIDDK NIH HHS/United States
- R01 CA150214/CA/NCI NIH HHS/United States
- R01 CA127641/CA/NCI NIH HHS/United States
- R01-CA39687/CA/NCI NIH HHS/United States
- R01-DK52825/DK/NIDDK NIH HHS/United States
- R01-CA140416/CA/NCI NIH HHS/United States
- U54 CA113001/CA/NCI NIH HHS/United States
- R01-CA108325/CA/NCI NIH HHS/United States
- P01 CA104177/CA/NCI NIH HHS/United States
- R01 CA140416/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

