Brivanib, a dual FGF/VEGF inhibitor, is active both first and second line against mouse pancreatic neuroendocrine tumors developing adaptive/evasive resistance to VEGF inhibition
- PMID: 21622725
- PMCID: PMC3156934
- DOI: 10.1158/1078-0432.CCR-10-2847
Brivanib, a dual FGF/VEGF inhibitor, is active both first and second line against mouse pancreatic neuroendocrine tumors developing adaptive/evasive resistance to VEGF inhibition
Abstract
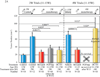
Purpose: Preclinical trials of a mouse model of pancreatic neuroendocrine tumors (PNET) were conducted to determine whether dual FGF/VEGF pathway inhibition with brivanib can improve first-line efficacy in comparison with VEGF inhibitors lacking fibroblast growth factor (FGF)-inhibitory activity and to characterize second-line brivanib activity before and after the onset of evasive resistance to VEGF-selective therapy.
Experimental design: An anti-VEGFR2 monoclonal antibody (DC101), an inhibitor of FGF signaling (FGF ligand trap), sorafenib, and brivanib were comparatively evaluated in first-line monotherapy in short and longer term fixed endpoint intervention trials in the RIP-Tag2 mouse model of PNET. Brivanib was also tested second line aiming to block adaptive resistance to selective VEGF therapies, assessing tumor growth, vascularity, hypoxia, invasion, and metastasis. The effects of initiating second-line brivanib therapy prior to or following overt relapse on sorafenib therapy were compared in overall survival trials to first-line therapies.
Results: Brivanib produced enduring tumor stasis and angiogenic blockade, both first and second line following the failure of DC101 or sorafenib. Overall survival was significantly extended by brivanib versus sorafenib, both first-line and when second-line therapy was initiated prior to sorafenib failure; second-line brivanib was less beneficial when initiated later, after the initiation of revascularization and incipient tumor progression.
Conclusions: Brivanib holds promise and deserves consideration for clinical evaluation as an antiangiogenic therapy, both in the context of impending failures of VEGF-selective therapy and in a first-line setting aiming to limit the adaptive response to VEGF inhibitors that results in evasive resistance.
©2011 AACR.
Conflict of interest statement
Ian Walters is an employee of Bristol-Myers Squibb. Douglas Hanahan is a consultant to Onyx Pharmaceutics, which co-markets sorafenib. Elizabeth Allen has no potential conflict of interest.
Figures








Comment in
-
Overcoming antiangiogenic resistance.Clin Cancer Res. 2011 Aug 15;17(16):5217-9. doi: 10.1158/1078-0432.CCR-11-1219. Epub 2011 Aug 9. Clin Cancer Res. 2011. PMID: 21828238
References
-
- Eriksson B. New drugs in neuroendocrine tumors: rising of new therapeutic philosophies? Curr Opin Oncol. 2010 Jul;22(4):381–386. - PubMed
-
- Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69(2):223–240. - PubMed
-
- Tomillero A, Moral MA. Gateways to clinical trials. Methods Find Exp Clin Pharmacol. 2009 Jul–Aug;31(6):397–417. - PubMed
-
- Huynh H. Tyrosine kinase inhibitors to treat liver cancer. Expert Opin Emerg Drugs. 2010 Feb 3; - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

