Phosphorylation of Spo0A by the histidine kinase KinD requires the lipoprotein med in Bacillus subtilis
- PMID: 21622736
- PMCID: PMC3147521
- DOI: 10.1128/JB.05199-11
Phosphorylation of Spo0A by the histidine kinase KinD requires the lipoprotein med in Bacillus subtilis
Abstract
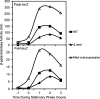
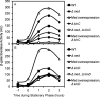
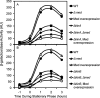
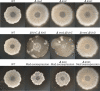
The response regulatory protein Spo0A of Bacillus subtilis is activated by phosphorylation by multiple histidine kinases via a multicomponent phosphorelay. Here we present evidence that the activity of one of the kinases, KinD, depends on the lipoprotein Med, a mutant of which has been known to cause a cannibalism phenotype. We show that the absence of Med impaired and the overproduction of Med stimulated the transcription of two operons (sdp and skf) involved in cannibalism whose transcription is known to depend on Spo0A in its phosphorylated state (Spo0A∼P). Further, these effects of Med were dependent on KinD but not on kinases KinA, KinB, and KinC. Additionally, we show that deletion or overproduction of Med impaired or enhanced, respectively, biofilm formation and that these effects, too, depended specifically on KinD. Finally, we report that overproduction of Med bypassed the dominant negative effect on transcription of sdp of a truncated KinD retaining the transmembrane segments but lacking the kinase domain. We propose that Med directly or indirectly interacts with KinD in the cytoplasmic membrane and that this interaction is required for KinD-dependent phosphorylation of Spo0A.
Figures
References
-
- Burbulys D., Trach K. A., Hoch J. A. 1991. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 64:545–552 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

