Assembly of minicellulosomes on the surface of Bacillus subtilis
- PMID: 21622797
- PMCID: PMC3147385
- DOI: 10.1128/AEM.02599-10
Assembly of minicellulosomes on the surface of Bacillus subtilis
Retraction in
-
Retraction for Anderson et al., "Assembly of Minicellulosomes on the Surface of Bacillus subtilis".Appl Environ Microbiol. 2018 Jan 31;84(4):e02429-17. doi: 10.1128/AEM.02429-17. Print 2018 Feb 15. Appl Environ Microbiol. 2018. PMID: 29386191 Free PMC article. No abstract available.
Abstract
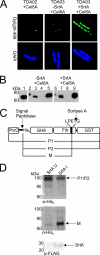
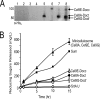
To cost-efficiently produce biofuels, new methods are needed to convert lignocellulosic biomass into fermentable sugars. One promising approach is to degrade biomass using cellulosomes, which are surface-displayed multicellulase-containing complexes present in cellulolytic Clostridium and Ruminococcus species. In this study we created cellulolytic strains of Bacillus subtilis that display one or more cellulase enzymes. Proteins containing the appropriate cell wall sorting signal are covalently anchored to the peptidoglycan by coexpressing them with the Bacillus anthracis sortase A (SrtA) transpeptidase. This approach was used to covalently attach the Cel8A endoglucanase from Clostridium thermocellum to the cell wall. In addition, a Cel8A-dockerin fusion protein was anchored on the surface of B. subtilis via noncovalent interactions with a cell wall-attached cohesin module. We also demonstrate that it is possible to assemble multienzyme complexes on the cell surface. A three-enzyme-containing minicellulosome was displayed on the cell surface; it consisted of a cell wall-attached scaffoldin protein noncovalently bound to three cellulase-dockerin fusion proteins that were produced in Escherichia coli. B. subtilis has a robust genetic system and is currently used in a wide range of industrial processes. Thus, grafting larger, more elaborate minicellulosomes onto the surface of B. subtilis may yield cellulolytic bacteria with increased potency that can be used to degrade biomass.
Figures



References
-
- Bayer E. A., Morag E., Lamed R. 1994. The cellulosome—a treasure-trove for biotechnology. Trends Biotechnol. 12: 379–386 - PubMed
-
- Bayer E. A., Shimon L. J., Shoham Y., Lamed R. 1998. Cellulosomes—structure and ultrastructure. J. Struct. Biol. 124: 221–234 - PubMed
-
- Bron S. 1990. Plasmids, p. 75–139 In Harwood C. R., Cutting S. M. (ed.), Molecular biological methods for bacillus. John Wiley and Sons, Chichester, United Kingdom
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

