Cytotoxic CD4+ T cell responses to EBV contrast with CD8 responses in breadth of lytic cycle antigen choice and in lytic cycle recognition
- PMID: 21622860
- PMCID: PMC3154640
- DOI: 10.4049/jimmunol.1100590
Cytotoxic CD4+ T cell responses to EBV contrast with CD8 responses in breadth of lytic cycle antigen choice and in lytic cycle recognition
Abstract
EBV, a B lymphotropic herpesvirus, encodes two immediate early (IE)-, >30 early (E)-, and >30 late (L)-phase proteins during its replication (lytic) cycle. Despite this, lytic Ag-induced CD8 responses are strongly skewed toward IE and a few E proteins only, all expressed before HLA I presentation is blocked in lytically infected cells. For comparison, we examined CD4(+) T cell responses to eight IE, E, or L proteins, screening 14 virus-immune donors to overlapping peptide pools in IFN-γ ELISPOT assays, and established CD4(+) T cell clones against 12 defined epitopes for target-recognition assays. We found that the lytic Ag-specific CD4(+) T cell response differs radically from its CD8 counterpart in that it is widely distributed across IE, E, and L Ag targets, often with multiple reactivities detectable per donor and with IE, E, or L epitope responses being numerically dominant, and that all CD4(+) T cell clones, whether IE, E, or L epitope-specific, show strong recognition of EBV-transformed B cell lines, despite the lines containing only a small fraction of lytically infected cells. Efficient recognition occurs because lytic Ags are released into the culture and are acquired and processed by neighboring latently infected cells. These findings suggested that lytic Ag-specific CD4 responses are driven by a different route of Ag display than drives CD8 responses and that such CD4 effectors could be therapeutically useful against EBV-driven lymphoproliferative disease lesions, which contain similarly small fractions of EBV-transformed cells entering the lytic cycle.
Figures
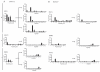



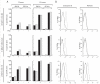
 ). (B) Flow cytometric analyses of intracellular perforin and granzyme B expression in fixed and permeabilised CD4+ T cell clones.
). (B) Flow cytometric analyses of intracellular perforin and granzyme B expression in fixed and permeabilised CD4+ T cell clones.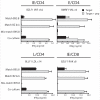

References
-
- Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25:587–617. - PubMed
-
- Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative disorders. Annu Rev Med. 2005;56:29–44. - PubMed
-
- Katz BZ, Raab-Traub N, Miller G. Latent and replicating forms of Epstein-Barr virus DNA in lymphomas and lymphoproliferative diseases. J Infect Dis. 1989;160:589–598. - PubMed
-
- Rea D, Fourcade C, Leblond V, Rowe M, Joab I, Edelman L, Bitker MO, Gandjbakhch I, Suberbielle C, Farcet JP, et al. Patterns of Epstein-Barr virus latent and replicative gene expression in Epstein-Barr virus B cell lymphoproliferative disorders after organ transplantation. Transplantation. 1994;58:317–324. - PubMed
-
- Rickinson A, Kieff E. Epstein-Barr virus. In: Fields B, Knipe D, Howley P, Chanock R, Melnick J, editors. Fields Virology. Lippincott, Williams & Wilkins; Philadelphia: 2007. pp. 2655–2700.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

