Genetic dissection of late-life fertility in Caenorhabditis elegans
- PMID: 21622982
- PMCID: PMC3148761
- DOI: 10.1093/gerona/glr089
Genetic dissection of late-life fertility in Caenorhabditis elegans
Abstract
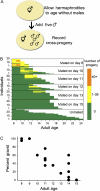
The large post-reproductive life span reported for the free-living hermaphroditic nematode, Caenorhabditis elegans, which lives for about 10 days after its 5-day period of self-reproduction, seems at odds with evolutionary theory. Species with long post-reproductive life spans such as mammals are sometimes explained by a need for parental care or transfer of information. This does not seem a suitable explanation for C elegans. Previous reports have shown that C elegans can regain fertility when mated after the self-fertile period but did not report the functional limits. Here, we report the functional life span of the C elegans germ line when mating with males. We show that C elegans can regain fertility late in life (significantly later than in previous reports) and that the end of this period corresponds quite well to its 3-week total life span. Genetic analysis reveals that late-life fertility is controlled by conserved pathways involved with aging and dietary restriction.
Figures




References
-
- Kirkwood TB, Shanley DP. The connections between general and reproductive senescence and the evolutionary basis of menopause. Ann N Y Acad Sci. 2010;1204:21–29. - PubMed
-
- Hamilton WD. The moulding of senescence by natural selection. J Theor Biol. 1966;12:12–45. - PubMed
-
- Sgro CM, Partridge L. A delayed wave of death from reproduction in Drosophila. Science. 1999;286:2521–2524. - PubMed
-
- Cohen AA. Female post-reproductive lifespan: a general mammalian trait. Biol Rev Camb Philos Soc. 2004;79:733–750. - PubMed
-
- Kirkwood TB. Evolution of ageing. Nature. 1977;270:301–304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

