Outcome of standardized treatment for patients with MDR-TB from Tamil Nadu, India
- PMID: 21623039
- PMCID: PMC3121285
Outcome of standardized treatment for patients with MDR-TB from Tamil Nadu, India
Abstract
Background & objectives: Programmatic management of MDR-TB using a standardized treatment regimen (STR) is being implemented under the Revised National Tuberculosis Control Programme (RNTCP) in India. This study was undertaken to analyse the outcomes of MDR-TB patients treated at the Tuberculosis Research Centre, Chennai, with the RNTCP recommended 24 months STR, under programmatic conditions.
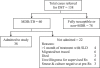
Methods: Patients failed to the category II re-treatment regimen and confirmed to have MDR-TB, were treated with the RNTCP's STR in a prospective field trial on a predominantly ambulatory basis. Thirty eight patients were enrolled to the trial from June 2006 to September 2007.
Results: Time to culture conversion was two months or less for 82 per cent of patients. Culture conversion rates at 3 and 6 months were 84 and 87 per cent respectively. At the end of treatment, 25 (66%) were cured, 5 defaulted, 3 died and 5 failed. At 24 months, 30 (79%) patients, including 5 defaulters, remained culture negative for more than 18 months. Twenty two (58%) patients reported adverse drug reactions (ADRs) which required dose reduction or termination of the offending drug. No patient had XDR-TB initially, but 2 failure cases emerged as XDR-TB during treatment.
Interpretation & conclusions: Outcomes of this small group of MDR-TB patients treated with the RNTCP's STR is encouraging in this setting. Close attention needs to be paid to ensure adherence, and to the timely recognition and treatment of ADRs.
Figures
References
-
- Geneva: WHO; 2009. World Health Organization (WHO). Global tuberculosis control 2009: epidemiology, strategy, financing. WHO/HTM/TB/2009.411.
-
- DOTS-plus guidelines. New Delhi: CTD; 2006. Central TB Division (CTD), Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India.
-
- Singla R, Sarin R, Khalid UK, Mathuria K, Singla N, Jaiswal A, et al. Seven-year DOTS-Plus pilot experience in India: Results, constraints and issues. Int J Tuberc Lung Dis. 2009;13:976–81. - PubMed
-
- Allen B, Baker FJ. London: Butterworth; 1968. Mycobacteria: isolation, identification and sensitivity testing.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources