Pituitary adenylate cyclase-activating peptide induces long-lasting neuroprotection through the induction of activity-dependent signaling via the cyclic AMP response element-binding protein-regulated transcription co-activator 1
- PMID: 21623792
- PMCID: PMC3557719
- DOI: 10.1111/j.1471-4159.2011.07330.x
Pituitary adenylate cyclase-activating peptide induces long-lasting neuroprotection through the induction of activity-dependent signaling via the cyclic AMP response element-binding protein-regulated transcription co-activator 1
Abstract
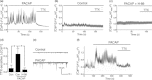
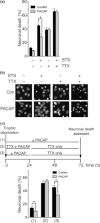
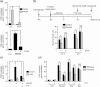
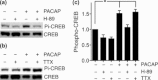
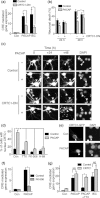
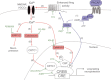
Pituitary adenylate cyclase-activating peptide (PACAP) is a neuroprotective peptide which exerts its effects mainly through the cAMP-protein kinase A (PKA) pathway. Here, we show that in cortical neurons, PACAP-induced PKA signaling exerts a major part of its neuroprotective effects indirectly, by triggering action potential (AP) firing. Treatment of cortical neurons with PACAP induces a rapid and sustained PKA-dependent increase in AP firing and associated intracellular Ca(2+) transients, which are essential for the anti-apoptotic actions of PACAP. Transient exposure to PACAP induces long-lasting neuroprotection in the face of apoptotic insults which is reliant on AP firing and the activation of cAMP response element (CRE) binding protein (CREB)-mediated gene expression. Although direct, activity-independent PKA signaling is sufficient to trigger phosphorylation on CREB's activating serine-133 site, this is insufficient for activation of CREB-mediated gene expression. Full activation is dependent on CREB-regulated transcription co-activator 1 (CRTC1), whose PACAP-induced nuclear import is dependent on firing activity-dependent calcineurin signaling. Over-expression of CRTC1 is sufficient to rescue PACAP-induced CRE-mediated gene expression in the face of activity-blockade, while dominant negative CRTC1 interferes with PACAP-induced, CREB-mediated neuroprotection. Thus, the enhancement of AP firing may play a significant role in the neuroprotective actions of PACAP and other adenylate cyclase-coupled ligands.
© 2011 The Authors. Journal of Neurochemistry © 2011 International Society for Neurochemistry.
Figures
References
-
- Atlasz T, Szabadfi K, Kiss P, et al. Pituitary adenylate cyclase activating polypeptide in the retina: focus on the retinoprotective effects. Ann. N Y Acad. Sci. 2010;1200:128–139. - PubMed
-
- Aubert N, Falluel-Morel A, Vaudry D, et al. PACAP and C2-ceramide generate different AP-1 complexes through a MAP-kinase-dependent pathway: involvement of c-Fos in PACAP-induced Bcl-2 expression. J. Neurochem. 2006;99:1237–1250. - PubMed
-
- Bading H, Greenberg ME. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991;253:912–914. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

