Integration of transient receptor potential canonical channels with lipids
- PMID: 21624095
- PMCID: PMC3734623
- DOI: 10.1111/j.1748-1716.2011.02311.x
Integration of transient receptor potential canonical channels with lipids
Abstract
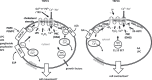
Transient receptor potential canonical (TRPC) channels are the canonical (C) subset of the TRP proteins, which are widely expressed in mammalian cells. They are thought to be primarily involved in determining calcium and sodium entry and have wide-ranging functions that include regulation of cell proliferation, motility and contraction. The channels are modulated by a multiplicity of factors, putatively existing as integrators in the plasma membrane. This review considers the sensitivities of TRPC channels to lipids that include diacylglycerols, phosphatidylinositol bisphosphate, lysophospholipids, oxidized phospholipids, arachidonic acid and its metabolites, sphingosine-1-phosphate, cholesterol and some steroidal derivatives and other lipid factors such as gangliosides. Promiscuous and selective lipid sensing have been detected. There appear to be close working relationships with lipids of the phospholipase C and A(2) enzyme systems, which may enable integration with receptor signalling and membrane stretch. There are differences in the properties of each TRPC channel that are further complicated by TRPC heteromultimerization. The lipids modulate activity of the channels or insertion in the plasma membrane. Lipid microenvironments and intermediate sensing proteins have been described that include caveolae, G protein signalling, SEC14-like and spectrin-type domains 1 (SESTD1) and podocin. The data suggest that lipid sensing is an important aspect of TRPC channel biology enabling integration with other signalling systems.
© 2011 The Author. Acta Physiologica © 2011 Scandinavian Physiological Society.
Figures
References
-
- Ahmmed GU, Mehta D, Vogel S, Holinstat M, Paria BC, Tiruppathi C, Malik AB. Protein kinase C alpha phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J Biol Chem. 2004;279:20941–20949. - PubMed
-
- Aires V, Hichami A, Boulay G, Khan NA. Activation of TRPC6 calcium channels by diacylglycerol (DAG)-containing arachidonic acid: a comparative study with DAG-containing docosahexaenoic acid. Biochimie. 2007;89:926–937. - PubMed
-
- Alicia S, Angelica Z, Carlos S, Alfonso S, Vaca L. STIM1 converts TRPC1 from a receptor-operated to a store-operated channel: moving TRPC1 in and out of lipid rafts. Cell Calcium. 2008;44:479–491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

