Analysis of timeliness of infectious disease reporting in the Netherlands
- PMID: 21624131
- PMCID: PMC3141442
- DOI: 10.1186/1471-2458-11-409
Analysis of timeliness of infectious disease reporting in the Netherlands
Abstract
Background: Timely reporting of infectious disease cases to public health authorities is essential to effective public health response. To evaluate the timeliness of reporting to the Dutch Municipal Health Services (MHS), we used as quantitative measures the intervals between onset of symptoms and MHS notification, and between laboratory diagnosis and notification with regard to six notifiable diseases.
Methods: We retrieved reporting data from June 2003 to December 2008 from the Dutch national notification system for shigellosis, EHEC/STEC infection, typhoid fever, measles, meningococcal disease, and hepatitis A virus (HAV) infection. For each disease, median intervals between date of onset and MHS notification were calculated and compared with the median incubation period. The median interval between date of laboratory diagnosis and MHS notification was similarly analysed. For the year 2008, we also investigated whether timeliness is improved by MHS agreements with physicians and laboratories that allow direct laboratory reporting. Finally, we investigated whether reports made by post, fax, or e-mail were more timely.
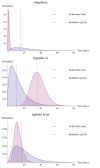
Results: The percentage of infectious diseases reported within one incubation period varied widely, between 0.4% for shigellosis and 90.3% for HAV infection. Not reported within two incubation periods were 97.1% of shigellosis cases, 76.2% of cases of EHEC/STEC infection, 13.3% of meningococcosis cases, 15.7% of measles cases, and 29.7% of typhoid fever cases. A substantial percentage of infectious disease cases was reported more than three days after laboratory diagnosis, varying between 12% for meningococcosis and 42% for shigellosis. MHS which had agreements with physicians and laboratories showed a significantly shorter notification time compared to MHS without such agreements.
Conclusions: Over the study period, many cases of the six notifiable diseases were not reported within two incubation periods, and many were reported more than three days after laboratory diagnosis. An increase in direct laboratory reporting of diagnoses to MHS would improve timeliness, as would the use of fax rather than post or e-mail. Automated reporting systems have to be explored in the Netherlands. Development of standardised and improved measures for timeliness is needed.
Figures


References
-
- Silk BJ, Berkelman RL. A review of strategies for enhancing the completeness of notifiable disease reporting. J Public Health Manag Pract. 2005;11:191–200. - PubMed
-
- Vogt RL, Spittle R, Cronquist A, Patnaik JL. Evaluation of the timeliness and completeness of a Web-based notifiable disease reporting system by a local health department. J Public Health Manag Pract. 2006;12:540–4. - PubMed
-
- World Health Organisation. Communicable Disease Surveillance and Response Systems. Guide to monitoring and evaluating. World Health Organisation; 2006. http://whqlibdoc.who.int/hq/2006/WHO_CDS_EPR_LYO_2006_2_eng.pdf
-
- Centers for Disease Control and Prevention. Updated guidelines for evaluating public health surveillance systems; Recommendations from the Guidelines Working Group. MMWR. 2001;50:1–35. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5013a1.htm - PubMed
-
- Ward M, Brandsema P, van Straten E, Bosman A. Electronic reporting improves timeliness and completeness of infectious disease notifications, The Netherlands, 2003. Eurosurveill. 2005;10:27–30. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

