Notch-dependent differentiation of adult airway basal stem cells
- PMID: 21624809
- PMCID: PMC3778678
- DOI: 10.1016/j.stem.2011.04.003
Notch-dependent differentiation of adult airway basal stem cells
Abstract
The epithelium lining the airways of the adult human lung is composed of ciliated and secretory cells together with undifferentiated basal cells (BCs). The composition and organization of this epithelium is severely disrupted in many respiratory diseases. However, little is known about the mechanisms controlling airway homeostasis and repair after epithelial damage. Here, we exploit the mouse tracheobronchial epithelium, in which BCs function as resident stem cells, as a genetically tractable model of human small airways. Using a reporter allele we show that the low level of Notch signaling at steady state is greatly enhanced during repair and the generation of luminal progenitors. Loss-of-function experiments show that Notch signaling is required for the differentiation, but not self-renewal, of BCs. Moreover, sustained Notch activation in BCs promotes their luminal differentiation, primarily toward secretory lineages. We also provide evidence that this function of Notch signaling is conserved in BCs from human airways.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




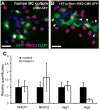
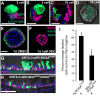
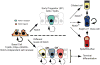
Comment in
-
Notch and basal cells take center stage during airway epithelial regeneration.Cell Stem Cell. 2011 Jun 3;8(6):597-8. doi: 10.1016/j.stem.2011.05.008. Cell Stem Cell. 2011. PMID: 21624798
References
-
- Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med. 1998;157:2000–2006. - PubMed
-
- Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–670. - PubMed
-
- Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR, Lindeman GJ, Visvader JE. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3:429–441. - PubMed
-
- Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24:2437–2447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

