Invariant NKT cells are required for airway inflammation induced by environmental antigens
- PMID: 21624935
- PMCID: PMC3173256
- DOI: 10.1084/jem.20102229
Invariant NKT cells are required for airway inflammation induced by environmental antigens
Abstract
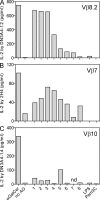
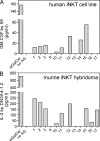
Invariant NKT cells (iNKT cells) are a unique subset of T lymphocytes that rapidly carry out effector functions. In this study, we report that a majority of sterile house dust extracts (HDEs) tested contained antigens capable of activating mouse and human iNKT cells. HDEs had adjuvant-like properties in an ovalbumin (OVA)-induced asthma model, which were dependent on Vα14i NKT cells, as vaccinated animals deficient for iNKT cells displayed significantly attenuated immune responses and airway inflammation. Furthermore, the administration of HDEs together with OVA mutually augmented the synthesis of cytokines by Vα14i NKT cells and by conventional CD4(+) T cells in the lung, demonstrating a profound immune response synergy for both Th2 cytokines and IL-17A. These data demonstrate that iNKT cell antigens are far more widely dispersed in the environment than previously anticipated. Furthermore, as the antigenic activity in different houses varied greatly, they further suggest that iNKT cell responses to ambient antigens, particular to certain environments, might promote sensitization to conventional respiratory allergens.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

