Hemophagocytosis causes a consumptive anemia of inflammation
- PMID: 21624938
- PMCID: PMC3173248
- DOI: 10.1084/jem.20102538
Hemophagocytosis causes a consumptive anemia of inflammation
Abstract
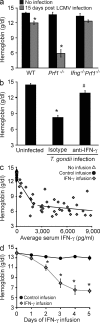
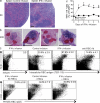
Cytopenias of uncertain etiology are commonly observed in patients during severe inflammation. Hemophagocytosis, the histological appearance of blood-eating macrophages, is seen in the disorder hemophagocytic lymphohistiocytosis and other inflammatory contexts. Although it is hypothesized that these phenomena are linked, the mechanisms facilitating acute inflammation-associated cytopenias are unknown. We report that interferon γ (IFN-γ) is a critical driver of the acute anemia observed during diverse microbial infections in mice. Furthermore, systemic exposure to physiologically relevant levels of IFN-γ is sufficient to cause acute cytopenias and hemophagocytosis. Demonstrating the significance of hemophagocytosis, we found that IFN-γ acts directly on macrophages in vivo to alter endocytosis and provoke blood cell uptake, leading to severe anemia. These findings define a unique pathological process of broad clinical and immunological significance, which we term the consumptive anemia of inflammation.
Figures
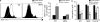
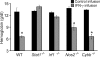
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

