PKCζ mediates disturbed flow-induced endothelial apoptosis via p53 SUMOylation
- PMID: 21624955
- PMCID: PMC3105539
- DOI: 10.1083/jcb.201010051
PKCζ mediates disturbed flow-induced endothelial apoptosis via p53 SUMOylation
Abstract
Atherosclerosis is readily observed in regions of blood vessels where disturbed blood flow (d-flow) is known to occur. A positive correlation between protein kinase C ζ (PKCζ) activation and d-flow has been reported, but the exact role of d-flow-mediated PKCζ activation in atherosclerosis remains unclear. We tested the hypothesis that PKCζ activation by d-flow induces endothelial cell (EC) apoptosis by regulating p53. We found that d-flow-mediated peroxynitrite (ONOO(-)) increased PKCζ activation, which subsequently induced p53 SUMOylation, p53-Bcl-2 binding, and EC apoptosis. Both d-flow and ONOO(-) increased the association of PKCζ with protein inhibitor of activated STATy (PIASy) via the Siz/PIAS-RING domain (amino acids 301-410) of PIASy, and overexpression of this domain of PIASy disrupted the PKCζ-PIASy interaction and PKCζ-mediated p53 SUMOylation. En face confocal microscopy revealed increases in nonnuclear p53 expression, nitrotyrosine staining, and apoptosis in aortic EC located in d-flow areas in wild-type mice, but these effects were significantly decreased in p53(-/-) mice. We propose a novel mechanism for p53 SUMOylation mediated by the PKCζ-PIASy interaction during d-flow-mediated EC apoptosis, which has potential relevance to early events of atherosclerosis.
Figures
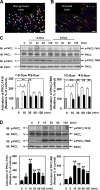




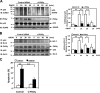




Comment in
-
Disturbed flow: p53 SUMOylation in the turnover of endothelial cells.J Cell Biol. 2011 May 30;193(5):805-7. doi: 10.1083/jcb.201104140. J Cell Biol. 2011. PMID: 21624951 Free PMC article.
References
-
- Akaike M., Che W., Marmarosh N.L., Ohta S., Osawa M., Ding B., Berk B.C., Yan C., Abe J.. 2004. The hinge-helix 1 region of peroxisome proliferator-activated receptor gamma1 (PPARgamma1) mediates interaction with extracellular signal-regulated kinase 5 and PPARgamma1 transcriptional activation: involvement in flow-induced PPARgamma activation in endothelial cells. Mol. Cell. Biol. 24:8691–8704. 10.1128/MCB.24.19.8691-8704.2004 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

