Partial netrin-1 deficiency aggravates acute kidney injury
- PMID: 21625583
- PMCID: PMC3098227
- DOI: 10.1371/journal.pone.0014812
Partial netrin-1 deficiency aggravates acute kidney injury
Abstract
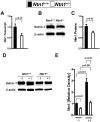
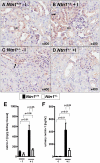
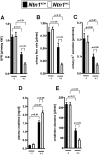
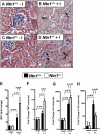
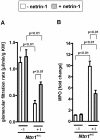
The netrin family of secreted proteins provides migrational cues in the developing central nervous system. Recently, netrins have also been shown to regulate diverse processes beyond their functions in the brain, incluing the ochrestration of inflammatory events. Particularly netrin-1 has been implicated in dampening hypoxia-induced inflammation. Here, we hypothesized an anti-inflammatory role of endogenous netrin-1 in acute kidney injury (AKI). As homozygous deletion of netrin-1 is lethal, we studied mice with partial netrin-1 deletion (Ntn-1(+/-) mice) as a genetic model. In fact, Ntn-1(+/-) mice showed attenuated Ntn-1 levels at baseline and following ischemic AKI. Functional studies of AKI induced by 30 min of renal ischemia and reperfusion revealed enhanced kidney dysfunction in Ntn-1(+/-) mice as assessed by measurements of glomerular filtration, urine flow rate, urine electrolytes, serum creatinine and creatinine clearance. Consistent with these findings, histological studies indicated a more severe degree kidney injury. Similarly, elevations of renal and systemic inflammatory markers were enhanced in mice with partial netrin-1 deficiency. Finally, treatment of Ntn-1(+/-) mice with exogenous netrin-1 restored a normal phenotype during AKI. Taking together, these studies implicate endogenous netrin-1 in attenuating renal inflammation during AKI.
Conflict of interest statement
Figures


References
-
- Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159–169. - PubMed
-
- Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797–805. - PubMed
-
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. - PubMed
-
- Gelman S. The pathophysiology of aortic cross-clamping and unclamping. Anesthesiology. 1995;82:1026–1060. - PubMed
-
- Mehta RL. Acute renal failure and cardiac surgery: marching in place or moving ahead? J Am Soc Nephrol. 2005;16:12–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

