Tumor immunotherapy using adenovirus vaccines in combination with intratumoral doses of CpG ODN
- PMID: 21626029
- PMCID: PMC4358758
- DOI: 10.1007/s00262-011-1038-y
Tumor immunotherapy using adenovirus vaccines in combination with intratumoral doses of CpG ODN
Abstract
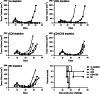
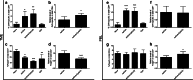
The combination of viral vaccination with intratumoral (IT) administration of CpG ODNs is yet to be investigated as an immunotherapeutic treatment for solid tumors. Here, we show that such a treatment regime can benefit survival of tumor-challenged mice. C57BL/6 mice bearing ovalbumin (OVA)-expressing EG.7 thymoma tumors were therapeutically vaccinated with adenovirus type 5 encoding OVA (Ad5-OVA), and the tumors subsequently injected with the immunostimulatory TLR9 agonist, CpG-B ODN 1826 (CpG), 4, 7, 10, and 13 days later. This therapeutic combination resulted in enhanced mean survival times that were more than 3.5× longer than naïve mice, and greater than 40% of mice were cured and capable of resisting subsequent tumor challenge. This suggests that an adaptive immune response was generated. Both Ad5-OVA and Ad5-OVA + CpG IT treatments led to significantly increased levels of H-2 K(b)-OVA-specific CD8+ lymphocytes in the peripheral blood and intratumorally. Lymphocyte depletion studies performed in vivo implicated both NK cells and CD8+ lymphocytes as co-contributors to the therapeutic effect. Analysis of tumor infiltrating lymphocytes (TILs) on day 12 post-tumor challenge revealed that mice treated with Ad5-OVA + CpG IT possessed a significantly reduced percentage of regulatory T lymphocytes (Tregs) within the CD4+ lymphocyte population, compared with TILs isolated from mice treated with Ad5-OVA only. In addition, the proportion of CD8+ TILs that were OVA-specific was reproducibly higher in the mice treated with Ad5-OVA + CpG IT compared with other treatment groups. These findings highlight the therapeutic potential of combining intratumoral CpG and vaccination with virus encoding tumor antigen.
Figures



References
-
- Farkas AM, Finn OJ (2010) Vaccines based on abnormal self-antigens as tumor-associated antigens: immune regulation. Semin Immunol - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

