Biomedical applications of tetrazine cycloadditions
- PMID: 21627112
- PMCID: PMC3166440
- DOI: 10.1021/ar200037t
Biomedical applications of tetrazine cycloadditions
Abstract
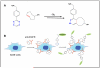
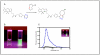
Disease mechanisms are increasingly being resolved at the molecular level. Biomedical success at this scale creates synthetic opportunities for combining specifically designed orthogonal reactions in applications such as imaging, diagnostics, and therapy. For practical reasons, it would be helpful if bioorthogonal coupling reactions proceeded with extremely rapid kinetics (k > 10(3) M(-1) s(-1)) and high specificity. Improving kinetics would minimize both the time and amount of labeling agent required to maintain high coupling yields. In this Account, we discuss our recent efforts to design extremely rapid bioorthogonal coupling reactions between tetrazines and strained alkenes. These selective reactions were first used to covalently couple conjugated tetrazine near-infrared-emitting fluorophores to dienophile-modifed extracellular proteins on living cancer cells. Confocal fluorescence microscopy demonstrated efficient and selective labeling, and control experiments showed minimal background fluorescence. Multistep techniques were optimized to work with nanomolar concentrations of labeling agent over a time scale of minutes: the result was successful real-time imaging of covalent modification. We subsequently discovered fluorogenic probes that increase in fluorescence intensity after the chemical reaction, leading to an improved signal-to-background ratio. Fluorogenic probes were used for intracellular imaging of dienophiles. We further developed strategies to react and image chemotherapeutics, such as trans-cyclooctene taxol analogues, inside living cells. Because the coupling partners are small molecules (<300 Da), they offer unique steric advantages in multistep amplification. We also describe recent success in using tetrazine reactions to label biomarkers on cells with magneto-fluorescent nanoparticles. Two-step protocols that use bioorthogonal chemistry can significantly amplify signals over both one-step labeling procedures as well as two-step procedures that use more sterically hindered biotin-avidin interactions. Nanoparticles can be detected with fluorescence or magnetic resonance techniques. These strategies are now being routinely used on clinical samples for biomarker profiling to predict malignancy and patient outcome. Finally, we discuss recent results with tetrazine reactions used for in vivo molecular imaging applications. Rapid tetrazine cycloadditions allow modular labeling of small molecules with the most commonly used positron emission tomography isotope, (18)F. Additionally, recent work has applied this reaction directly in vivo for the pretargeted imaging of solid tumors. Future work with tetrazine cycloadditions will undoubtedly lead to optimized protocols, improved probes, and additional biomedical applications.
© 2011 American Chemical Society
Figures








References
-
- Prescher JA, Bertozzi CR. Chemistry in living systems. Nat Chem Biol. 2005;1:13–21. - PubMed
-
- Speers AE, Adam GC, Cravatt BF. Activity-based protein profiling in vivo using a copper(i)-catalyzed azide-alkyne [3 + 2] cycloaddition. J Am Chem Soc. 2003;125:4686–4687. - PubMed
-
- Lewis WG, et al. Click chemistry in situ: acetylcholinesterase as a reaction vessel for the selective assembly of a femtomolar inhibitor from an array of building blocks. Angew. Chem. Int. Ed. 2002;41:1053–1057. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

