Diastereo- and enantioselective ruthenium-catalyzed hydrohydroxyalkylation of 2-silyl-butadienes: carbonyl syn-crotylation from the alcohol oxidation level
- PMID: 21627316
- PMCID: PMC3131435
- DOI: 10.1021/ja2046028
Diastereo- and enantioselective ruthenium-catalyzed hydrohydroxyalkylation of 2-silyl-butadienes: carbonyl syn-crotylation from the alcohol oxidation level
Abstract
Exposure of alcohols 2a-2j to 2-silyl-butadienes in the presence of ruthenium complexes modified by (R)-SEGPHOS or (R)-DM-SEGPHOS results in redox-triggered generation of allylruthenium-aldehyde pairs, which combine to form products of carbonyl crotylation 4a-4j in the absence of stoichiometric byproducts and with high levels of syn-diastereo- and enantioselectivity. In the presence of isopropanol under otherwise identical conditions, aldehydes 3a-3j are converted to an equivalent set of adducts 4a-4j. Whereas reactions conducted using conventional heating require 48 h, microwave irradiation enables full conversion in only 4 h. Finally, as illustrated in the conversion of adduct 4a to compounds 6a and 6b, diastereoselective hydroboration-Suzuki cross-coupling with aryl and vinyl halides followed by Fleming-Tamao oxidation enables generation of anti,syn-stereotriads found in numerous polyketide natural products.
Figures


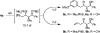

References
-
-
For selected reviews on polyketide natural products, see: O'Hagan D. The Polyketide Metabolites. Ellis Horwood; Chichester, U.K.: 1991. Rimando AM, Baerson SR, editors. Polyketides Biosynthesis, Biological Activity, and Genetic Engineering. American Chemical Society; Washington, DC: 2007. (ACS Symposium Series 955).
-
-
- Rohr J. Angew. Chem., Int. Ed. 2000;39:2847. - PubMed
-
-
For selected reviews on synthetic methods for polyketide construction, see: Paterson I, Doughty VA, Florence G, Gerlach K, McLeod MD, Scott JP, Trieselmann T. ACS Symp. Ser. 2001;783:195.Koskinen AMP, Karisalmi K. Chem. Soc. Rev. 2005;34:677.Yeung K-S, Paterson I. Chem. Rev. 2005;105:4237.Schetter B, Mahrwald R. Angew. Chem., Int. Ed. 2006;45:7506.Morris JC, Nicholas GM, Phillips AJ. Nat. Prod. Rep. 2007;24:87.Paterson I. Total Synthesis of Polyketides using Asymmetric Aldol Reactions. In: Christmann M, Bräse S, editors. Asymmetric Synthesis. 2nd Edition Wiley-VCH Verlag GmbH & Co; Weinheim, Germany: 2008. pp. 293–298.Paterson I, Findlay AD. Aust. J. Chem. 2009;62:624.
-
-
-
For enantioselective carbonyl additions of chirally modified crotylmetal reagents, see: Hoffmann RW, Ladner W. Tetrahedron Lett. 1979;20:4653.Brown HC, Bhat KS. J. Am. Chem. Soc. 1986;108:293.Brown HC, Bhat KS. J. Am. Chem. Soc. 1986;108:5919.Roush WR, Halterman RL. J. Am. Chem. Soc. 1986;108:294.Roush WR, Ando K, Powers DB, Palkowitz AD, Halterman RL. J. Am. Chem. Soc. 1990;112:6339.Garcia J, Kim B-M, Masamune S. J. Org. Chem. 1987;52:4831.Burgos CH, Canales E, Matos K, Soderquist JA. J. Am. Chem. Soc. 2005;127:8044.Riediker M, Duthaler RO. Angew. Chem., Int. Ed. Engl. 1989;28:494.Hafner A, Duthaler RO, Marti R, Rihs G, Rothe-Streit P, Schwarzenbach F. J. Am. Chem. Soc. 1992;114:2321.Panek JS, Yang M. J. Am. Chem. Soc. 1991;113:6594.Hu T, Takenaka N, Panek JS. J. Am. Chem. Soc. 2002;124:12806.Hackman BM, Lombardi PJ, Leighton JL. Org. Lett. 2004;6:4375.Kim H, Ho S, Leighton JL. J. Am. Chem. Soc. 2011;133:6517.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

