The orexin receptor OX(1)R in colon cancer: a promising therapeutic target and a new paradigm in G protein-coupled receptor signalling through ITIMs
- PMID: 21627633
- PMCID: PMC3372822
- DOI: 10.1111/j.1476-5381.2011.01510.x
The orexin receptor OX(1)R in colon cancer: a promising therapeutic target and a new paradigm in G protein-coupled receptor signalling through ITIMs
Abstract
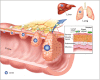
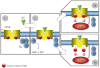
An exciting aspect of the heptahelical orexin receptor 1 (OX(1)R) has emerged recently, when it was shown that it drives apoptosis in human colon cancer cell lines. Here we review recent findings related to the role of OX(1)R in colorectal cancers and the unexpected mechanism whereby this G protein-coupled receptor works. The OX(1)R is aberrantly expressed at all steps of primary colorectal tumour progression and after local (lymph node) or distant (liver, lung) metastasis. No OX(1)R is detected in normal colonic epithelial cells. Treatment of human colon cancer cells in culture with orexins promotes robust apoptosis and subsequent reduction of growth including in cells that are resistant to 5-fluorouracil, the most commonly used drug in chemotherapy. When human colon cancer cells are xenografted in nude mice, treatment with orexins dramatically slows tumour growth and even reverses the development of established tumours. Thus, OX(1)R agonists might be novel candidates for colon cancer therapy. Activation of OX(1)R drives apoptosis through G(q) protein but independently of classical Gα(q) activation of phospholipase C. In fact, it is the freed βγ dimer of G(q) that plays a pivotal role by stimulating Src-tyrosine kinase. This results in phosphorylation of two immunoreceptor tyrosine-based inhibitory motifs (ITIM) in OX(1)R and subsequent recruitment by OX(1)R of the phosphotyrosine phosphatase SHP-2, which is activated thereby. Downstream events include release of cytochrome c from mitochondria and activation of caspase-3 and caspase-7. The role of ITIMs in OX(1)R-driven apoptosis represents a new paradigm of G protein-coupled receptor signalling.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures

References
-
- Ammoun S, Lindholm D, Woortz H, Akerman KE, Kukkonen JP. G-protein-coupled OX1 orexin/hctr-1 hypocretin receptors induce caspase-dependent and -independent cell death through p38 mitogen-/stress-activated protein kinase. J Biol Chem. 2006;281:834–842. - PubMed
-
- Bjorge JD, Jakymiv A, Fujita DJ. Selected glimpses into the activation and function of Src kinase. Oncogene. 2000;19:5620–5635. - PubMed
-
- Boss C, Brisbare-Roch C, Jenck F. Biomedical application of orexin/hypocretin receptor ligands in neuroscience. J Med Chem. 2009;52:891–903. - PubMed
-
- Bralet MP, Paule B, Adam R, Guettier C. Loss of epidermal growth factor receptor expression in lymph node and liver metastases of colon carcinoma. J Clin Oncol. 2005;23:5844–5845. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

