Phase II study of the combination carboplatin plus celecoxib in heavily pre-treated recurrent ovarian cancer patients
- PMID: 21627839
- PMCID: PMC3123659
- DOI: 10.1186/1471-2407-11-214
Phase II study of the combination carboplatin plus celecoxib in heavily pre-treated recurrent ovarian cancer patients
Abstract
Background: Cyclooxygenase-2 overexpression is associated with poor outcome and resistance to platinum-based chemotherapy in ovarian cancer. We evaluated the antitumor activity and safety of the combination carboplatin plus the COX-2 inhibitor celecoxib in recurrent heavily-treated OC patients.
Methods: Patients were administered oral celecoxib (400 mg/day) in combination with intravenous carboplatin (AUC5, q28). A Simon's two-stage design was employed.
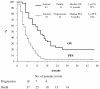
Results: 45 patients were enrolled: 23 (51.1%) presented platinum-resistance, and 27 (60%) had received at least 3 prior regimens for recurrence. The response rate was 28.9% with 3 complete and 10 partial responses (median duration of response = 6 months). Only one (0.4%) G4 non-febrile neutropenia was observed; G3 neutropenia, anemia, or thrombocytopenia, were observed in 2.5%, 1.7%, and 1.7% of the cycles, respectively. G3-4 vomiting was reported in only 1.7%, and 0.4% of the cycles were associated with G3 dyspepsia or diarrhea or constipation. Only one patient experienced G3 hypertension associated to G2 hypersensitivity reaction. No differences in baseline versus post-treatment Quality of Life scores were observed. Median progression free survival and overall survival were 5 and 13 months, respectively.
Conclusions: Celecoxib combined with carboplatin showed promising activity and it is well tolerated in heavily-treated recurrent ovarian cancer patients.
Trial registration number: NCT01124435 (ClinicalTrials.gov Identifier) and 935/03 (study ID numbers).
Figures
References
-
- Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, Wheeler S, Swart AM, Qian W, Torri V, Floriani I, Jayson G, Lamont A, Tropé C. ICON and AGO Collaborators. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–106. - PubMed
-
- Pfisterer J, Plante M, Vergote I, du Bois A, Hirte H, Lacave AJ, Wagner U, Stähle A, Stuart G, Kimmig R, Olbricht S, Le T, Emerich J, Kuhn W, Bentley J, Jackisch C, Lück HJ, Rochon J, Zimmermann AH, Eisenhauer E. AGO-OVAR; NCIC CTG; EORTC GCG. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–707. doi: 10.1200/JCO.2006.06.0913. - DOI - PubMed
-
- Ferrandina G, Ludovisi M, Lorusso D, Pignata S, Breda E, Savarese A, Del Medico P, Scaltriti L, Katsaros D, Priolo D, Scambia G. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol. 2008;26:890–6. doi: 10.1200/JCO.2007.13.6606. - DOI - PubMed
-
- Gordon AN, Tonda M, Sun S, Rackoff W. Doxil Study 30-49 Investigators. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol. 2004;95:1–8. doi: 10.1016/j.ygyno.2004.07.011. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials