Effect of weekly vitamin D supplements on mortality, morbidity, and growth of low birthweight term infants in India up to age 6 months: randomised controlled trial
- PMID: 21628364
- PMCID: PMC3104477
- DOI: 10.1136/bmj.d2975
Effect of weekly vitamin D supplements on mortality, morbidity, and growth of low birthweight term infants in India up to age 6 months: randomised controlled trial
Abstract
Objective: To investigate whether vitamin D supplementation can decrease the mortality and morbidity of low birthweight infants in low income countries.
Design: Randomised controlled trial.
Setting: Large government hospital in New Delhi, India.
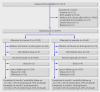
Participants: 2079 low birthweight infants born at term (>37 weeks' gestation).
Main outcome measures: Primary outcome was admission to hospital or death during the first six months of life. Main secondary outcome was growth.
Interventions: Weekly vitamin D supplements for six months at a dose of one recommended nutrient intake per day (35 µg/week). Infants were visited weekly at home for observed supplementation and were brought to the clinic monthly for clinical examination and anthropometric measurements.
Results: Between group differences were not significant for death or hospital admissions (92 among 1039 infants in the vitamin D group v 99 among 1040 infants in the placebo group; adjusted rate ratio 0.93, 95% confidence interval 0.68 to 1.29; P = 0.68), or referral to the outpatient clinic for moderate morbidity. Vitamin D supplementation resulted in better vitamin D status as assessed by plasma calcidiol levels at six months. In adjusted analyses, vitamin D treatment significantly increased standard deviation (z) scores at six months for weight, length, and arm circumference and decreased the proportion of children with stunted growth (length for age z score ≤ 2) or with arm circumference z scores of 2 or less.
Conclusion: A weekly dose of vitamin D resulted in better vitamin D status and benefited the classic vitamin D function of bone growth but did not decrease the incidence of severe morbidity or death among young low birthweight infants. Trial registration ClinicalTrials.gov NCT00415402.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
References
-
- Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81. - PubMed
-
- Van Etten E, Stoffels K, Gysemans C, Mathieu C, Overbergh L. Regulation of vitamin D homeostasis: implications for the immune system. Nutr Rev 2008;66(10 suppl 2):S125-34. - PubMed
-
- Roth DE, Shah R, Black RE, Baqui AH. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatr 2010;99:389-93. - PubMed
-
- Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamaki H, et al. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr 2007;86:714-7. - PubMed
-
- Gibney KB, MacGregor L, Leder K, Torresi J, Marshall C, Ebeling PR, et al. Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin Infect Dis 2008;46:443-6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical