Megakaryocytes differentially sort mRNAs for matrix metalloproteinases and their inhibitors into platelets: a mechanism for regulating synthetic events
- PMID: 21628401
- PMCID: PMC3158719
- DOI: 10.1182/blood-2010-12-324517
Megakaryocytes differentially sort mRNAs for matrix metalloproteinases and their inhibitors into platelets: a mechanism for regulating synthetic events
Abstract
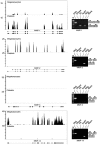
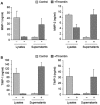
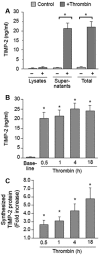
Megakaryocytes transfer a diverse and functional transcriptome to platelets during the final stages of thrombopoiesis. In platelets, these transcripts reflect the expression of their corresponding proteins and, in some cases, serve as a template for translation. It is not known, however, if megakaryocytes differentially sort mRNAs into platelets. Given their critical role in vascular remodeling and inflammation, we determined whether megakaryocytes selectively dispense transcripts for matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) into platelets. Next-generation sequencing (RNA-Seq) revealed that megakaryocytes express mRNA for 10 of the 24 human MMP family members. mRNA for all of these MMPs are present in platelets with the exception of MMP-2, 14, and 15. Megakaryocytes and platelets also express mRNA for TIMPs 1-3, but not TIMP-4. mRNA expression patterns predicted the presence and, in most cases, the abundance of each corresponding protein. Nonetheless, exceptions were observed: MMP-2 protein is present in platelets but not its transcript. In contrast, quiescent platelets express TIMP-2 mRNA but only traces of TIMP-2 protein. In response to activating signals, however, platelets synthesize significant amounts of TIMP-2 protein. These results demonstrate that megakaryocytes differentially express mRNAs for MMPs and TIMPs and selectively transfer a subset of these into platelets. Among the platelet messages, TIMP-2 serves as a template for signal-dependent translation.
Figures

Comment in
-
Platelets get the message.Blood. 2011 Aug 18;118(7):1712-3. doi: 10.1182/blood-2011-06-359802. Blood. 2011. PMID: 21852441 No abstract available.
-
Differential expression of MMP-2 and MMP-9 activity in megakaryocytes and platelets.Blood. 2011 Dec 8;118(24):6470-1; author reply 6471-3. doi: 10.1182/blood-2011-07-366195. Blood. 2011. PMID: 22161853 No abstract available.
References
-
- Italiano JE, Jr, Shivdasani RA. Megakaryocytes and beyond: the birth of platelets. J Thromb Haemost. 2003;1(6):1174–1182. - PubMed
-
- Junt T, Schulze H, Chen Z, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317(5845):1767–1770. - PubMed
-
- Italiano JE, Jr, Patel-Hett S, Hartwig JH. Mechanics of proplatelet elaboration. J Thromb Haemost. 2007;5(suppl 1):18–23. - PubMed
-
- van Nispen tot Pannerden H, de Haas F, Geerts W, Posthuma G, van Dijk S, Heijnen HF. The platelet interior revisited: electron tomography reveals tubular alpha-granule subtypes. Blood. 2010;116(7):1147–1156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

