Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress
- PMID: 21628424
- PMCID: PMC3113668
- DOI: 10.1242/jcs.080762
Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress
Abstract
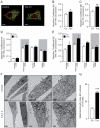
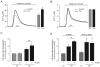
Increasing evidence indicates that endoplasmic reticulum (ER) stress activates the adaptive unfolded protein response (UPR), but that beyond a certain degree of ER damage, this response triggers apoptotic pathways. The general mechanisms of the UPR and its apoptotic pathways are well characterized. However, the metabolic events that occur during the adaptive phase of ER stress, before the cell death response, remain unknown. Here, we show that, during the onset of ER stress, the reticular and mitochondrial networks are redistributed towards the perinuclear area and their points of connection are increased in a microtubule-dependent fashion. A localized increase in mitochondrial transmembrane potential is observed only in redistributed mitochondria, whereas mitochondria that remain in other subcellular zones display no significant changes. Spatial re-organization of these organelles correlates with an increase in ATP levels, oxygen consumption, reductive power and increased mitochondrial Ca²⁺ uptake. Accordingly, uncoupling of the organelles or blocking Ca²⁺ transfer impaired the metabolic response, rendering cells more vulnerable to ER stress. Overall, these data indicate that ER stress induces an early increase in mitochondrial metabolism that depends crucially upon organelle coupling and Ca²⁺ transfer, which, by enhancing cellular bioenergetics, establishes the metabolic basis for the adaptation to this response.
Figures



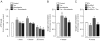

References
-
- Bach D., Pich S., Soriano F. X., Vega N., Baumgartner B., Oriola J., Daugaard J. R., Lloberas J., Camps M., Zierath J. R., et al. (2003). Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J. Biol. Chem. 278, 17190-17197 - PubMed
-
- Berridge M. J. (2002). The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium 32, 235-249 - PubMed
-
- Boldogh I. R., Pon L. A. (2006). Interactions of mitochondria with the actin cytoskeleton. Biochim. Biophys. Acta 1763, 450-462 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

