Quality control of mitochondrial proteostasis
- PMID: 21628427
- PMCID: PMC3119916
- DOI: 10.1101/cshperspect.a007559
Quality control of mitochondrial proteostasis
Abstract
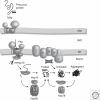
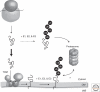
A decline in mitochondrial activity has been associated with aging and is a hallmark of many neurological diseases. Surveillance mechanisms acting at the molecular, organellar, and cellular level monitor mitochondrial integrity and ensure the maintenance of mitochondrial proteostasis. Here we will review the central role of mitochondrial chaperones and proteases, the cytosolic ubiquitin-proteasome system, and the mitochondrial unfolded response in this interconnected quality control network, highlighting the dual function of some proteases in protein quality control within the organelle and for the regulation of mitochondrial fusion and mitophagy.
Figures



References
-
- Anesti V, Scorrano L 2006. The relationship between mitochondrial shape and function and the cytoskeleton. Biochim Biophys Acta 1757: 692–699 - PubMed
-
- Arlt H, Tauer R, Feldmann H, Neupert W, Langer T 1996. The YTA10-12 complex, an AAA protease with chaperone-like activity in the inner membrane of mitochondria. Cell 85: 875–885 - PubMed
-
- Arnold I, Wagner-Ecker M, Ansorge W, Langer T 2006. Evidence for a novel mitochondria-to-nucleus signalling pathway in respiring cells lacking i-AAA protease and the ABC-transporter Mdl1. Gene 367: 74–88 - PubMed
-
- Augustin S, Nolden M, Muller S, Hardt O, Arnold I, Langer T 2005. Characterization of peptides released from mitochondria: Evidence for constant proteolysis and peptide efflux. J Biol Chem 280: 2691–2699 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
