Multiple BH3 mimetics antagonize antiapoptotic MCL1 protein by inducing the endoplasmic reticulum stress response and up-regulating BH3-only protein NOXA
- PMID: 21628457
- PMCID: PMC3137063
- DOI: 10.1074/jbc.M111.255828
Multiple BH3 mimetics antagonize antiapoptotic MCL1 protein by inducing the endoplasmic reticulum stress response and up-regulating BH3-only protein NOXA
Abstract
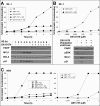
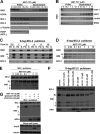
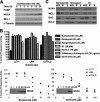
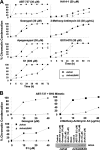
BH3 mimetics are small molecules designed or discovered to mimic the binding of BH3-only proteins to the hydrophobic groove of antiapoptotic BCL2 proteins. The selectivity of these molecules for BCL2, BCL-X(L), or MCL1 has been established in vitro; whether they inhibit these proteins in cells has not been rigorously investigated. In this study, we used a panel of leukemia cell lines to assess the ability of seven putative BH3 mimetics to inhibit antiapoptotic proteins in a cell-based system. We show that ABT-737 is the only BH3 mimetic that inhibits BCL2 as assessed by displacement of BAD and BIM from BCL2. The other six BH3 mimetics activate the endoplasmic reticulum stress response inducing ATF4, ATF3, and NOXA, which can then bind to and inhibit MCL1. In most cancer cells, inhibition of one antiapoptotic protein does not acutely induce apoptosis. However, by combining two BH3 mimetics, one that inhibits BCL2 and one that induces NOXA, apoptosis is induced within 6 h in a BAX/BAK-dependent manner. Because MCL1 is a major mechanism of resistance to ABT-737, these results suggest a novel strategy to overcome this resistance. Our findings highlight a novel signaling pathway through which many BH3 mimetics inhibit MCL1 and suggest the potential use of these agents as adjuvants in combination with various chemotherapy strategies.
Figures




References
-
- Walensky L. D. (2006) Cell Death Differ. 13, 1339–1350 - PubMed
-
- Hanahan D., Weinberg R. A. (2000) Cell 100, 57–70 - PubMed
-
- Oltersdorf T., Elmore S. W., Shoemaker A. R., Armstrong R. C., Augeri D. J., Belli B. A., Bruncko M., Deckwerth T. L., Dinges J., Hajduk P. J., Joseph M. K., Kitada S., Korsmeyer S. J., Kunzer A. R., Letai A., Li C., Mitten M. J., Nettesheim D. G., Ng S., Nimmer P. M., O'Connor J. M., Oleksijew A., Petros A. M., Reed J. C., Shen W., Tahir S. K., Thompson C. B., Tomaselli K. J., Wang B., Wendt M. D., Zhang H., Fesik S. W., Rosenberg S. H. (2005) Nature 435, 677–681 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

