Effects of vasoactive intestinal peptide genotype on circadian gene expression in the suprachiasmatic nucleus and peripheral organs
- PMID: 21628547
- PMCID: PMC3942163
- DOI: 10.1177/0748730411401740
Effects of vasoactive intestinal peptide genotype on circadian gene expression in the suprachiasmatic nucleus and peripheral organs
Abstract
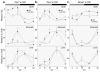
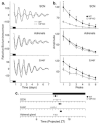
The neuropeptide vasoactive intestinal polypeptide (VIP) has emerged as a key candidate molecule mediating the synchronization of rhythms in clock gene expression within the suprachiasmatic nucleus (SCN). In addition, neurons expressing VIP are anatomically well positioned to mediate communication between the SCN and peripheral oscillators. In this study, we examined the temporal expression profile of 3 key circadian genes: Per1, Per2 , and Bmal1 in the SCN, the adrenal glands and the liver of mice deficient for the Vip gene (VIP KO), and their wild-type counterparts. We performed these measurements in mice held in a light/dark cycle as well as in constant darkness and found that rhythms in gene expression were greatly attenuated in the VIP-deficient SCN. In the periphery, the impact of the loss of VIP varied with the tissue and gene measured. In the adrenals, rhythms in Per1 were lost in VIP-deficient mice, while in the liver, the most dramatic impact was on the phase of the diurnal expression rhythms. Finally, we examined the effects of the loss of VIP on ex vivo explants of the same central and peripheral oscillators using the PER2::LUC reporter system. The VIP-deficient mice exhibited low amplitude rhythms in the SCN as well as altered phase relationships between the SCN and the peripheral oscillators. Together, these data suggest that VIP is critical for robust rhythms in clock gene expression in the SCN and some peripheral organs and that the absence of this peptide alters both the amplitude of circadian rhythms as well as the phase relationships between the rhythms in the SCN and periphery.
Figures

References
-
- Aton S, Block G, Tei H, Yamazaki S, Herzog E. Plasticity of circadian behavior and the suprachiasmatic nucleus following exposure to non-24-hour light cycles. J Biol Rhythms. 2004;19:198–207. - PubMed
-
- Balsalobre A, Brown S, Marcacci L, Tronche F, Kellendonk C, Reichardt H, Schütz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

