Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants
- PMID: 21628566
- PMCID: PMC3156158
- DOI: 10.1073/pnas.1100659108
Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants
Abstract
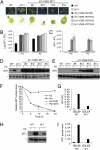
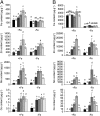
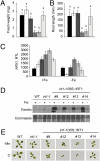
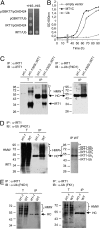
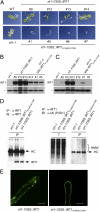
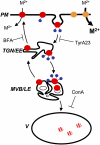
Plants take up iron from the soil using the iron-regulated transporter 1 (IRT1) high-affinity iron transporter at the root surface. Sophisticated regulatory mechanisms allow plants to tightly control the levels of IRT1, ensuring optimal absorption of essential but toxic iron. Here, we demonstrate that overexpression of Arabidopsis thaliana IRT1 leads to constitutive IRT1 protein accumulation, metal overload, and oxidative stress. IRT1 is unexpectedly found in trans-Golgi network/early endosomes of root hair cells, and its levels and localization are unaffected by iron nutrition. Using pharmacological approaches, we show that IRT1 cycles to the plasma membrane to perform iron and metal uptake at the cell surface and is sent to the vacuole for proper turnover. We also prove that IRT1 is monoubiquitinated on several cytosol-exposed residues in vivo and that mutation of two putative monoubiquitination target residues in IRT1 triggers stabilization at the plasma membrane and leads to extreme lethality. Together, these data suggest a model in which monoubiquitin-dependent internalization/sorting and turnover keep the plasma membrane pool of IRT1 low to ensure proper iron uptake and to prevent metal toxicity. More generally, our work demonstrates the existence of monoubiquitin-dependent trafficking to lytic vacuoles in plants and points to proteasome-independent turnover of plasma membrane proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Curie C, Briat JF. Iron transport and signaling in plants. Annu Rev Plant Biol. 2003;54:183–206. - PubMed
-
- Briat JF, et al. Cellular and molecular aspects of iron metabolism in plants. Biol Cell. 1995;84:69–81.
-
- Henriques R, et al. Knock-out of Arabidopsis metal transporter gene IRT1 results in iron deficiency accompanied by cell differentiation defects. Plant Mol Biol. 2002;50:587–597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

