Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts
- PMID: 21628574
- PMCID: PMC3116385
- DOI: 10.1073/pnas.1100816108
Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts
Abstract
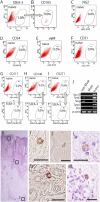
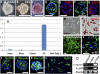
The stochastic and elite models have been proposed for the mechanism of induced pluripotent stem (iPS) cell generation. In this study we report a system that supports the elite model. We previously identified multilineage-differentiating stress-enduring (Muse) cells in human dermal fibroblasts that are characterized by stress tolerance, expression of pluripotency markers, self-renewal, and the ability to differentiate into endodermal-, mesodermal-, and ectodermal-lineage cells from a single cell. They can be isolated as stage-specific embryonic antigen-3/CD105 double-positive cells. When human fibroblasts were separated into Muse and non-Muse cells and transduced with Oct3/4, Sox2, Klf4, and c-Myc, iPS cells were generated exclusively from Muse cells but not from non-Muse cells. Although some colonies were formed from non-Muse cells, they were unlike iPS cells. Furthermore, epigenetic alterations were not seen, and some of the major pluripotency markers were not expressed for the entire period during iPS cell generation. These findings were confirmed further using cells transduced with a single polycistronic virus vector encoding all four factors. The results demonstrate that in adult human fibroblasts a subset of preexisting adult stem cells whose properties are similar in some respects to those of iPS cells selectively become iPS cells, but the remaining cells make no contribution to the generation of iPS cells. Therefore this system seems to fit the elite model rather than the stochastic model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kørbling M, Estrov Z. Adult stem cells for tissue repair - a new therapeutic concept? N Engl J Med. 2003;349:570–582. - PubMed
-
- Thomas ED. Landmarks in the development of hematopoietic cell transplantation. World J Surg. 2000;24:815–818. - PubMed
-
- Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
-
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Sullivan GJ, Bai Y, Fletcher J, Wilmut I. Induced pluripotent stem cells: Epigenetic memories and practical implications. Mol Hum Reprod. 2010;16:880–885. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

