In vivo diagnosis of murine pancreatic intraepithelial neoplasia and early-stage pancreatic cancer by molecular imaging
- PMID: 21628592
- PMCID: PMC3116402
- DOI: 10.1073/pnas.1100890108
In vivo diagnosis of murine pancreatic intraepithelial neoplasia and early-stage pancreatic cancer by molecular imaging
Abstract
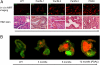
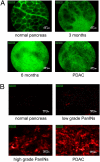
Pancreatic ductal adenocarcinoma (PDAC) is a fatal disease with poor patient outcome often resulting from late diagnosis in advanced stages. To date methods to diagnose early-stage PDAC are limited and in vivo detection of pancreatic intraepithelial neoplasia (PanIN), a preinvasive precursor of PDAC, is impossible. Using a cathepsin-activatable near-infrared probe in combination with flexible confocal fluorescence lasermicroscopy (CFL) in a genetically defined mouse model of PDAC we were able to detect and grade murine PanIN lesions in real time in vivo. Our diagnostic approach is highly sensitive and specific and proved superior to clinically established fluorescein-enhanced imaging. Translation of this endoscopic technique into the clinic should tremendously improve detection of pancreatic neoplasia, thus reforming management of patients at risk for PDAC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Schneider G, Siveke JT, Eckel F, Schmid RM. Pancreatic cancer: Basic and clinical aspects. Gastroenterology. 2005;128:1606–1625. - PubMed
-
- Furukawa H, et al. Clinicopathologic features of small pancreatic adenocarcinoma. A collective study. Cancer. 1996;78:986–990. - PubMed
-
- Agarwal B, Correa AM, Ho L. Survival in pancreatic carcinoma based on tumor size. Pancreas. 2008;36:e15–e20. - PubMed
-
- Shimada K, Sakamoto Y, Sano T, Kosuge T, Hiraoka N. Reappraisal of the clinical significance of tumor size in patients with pancreatic ductal carcinoma. Pancreas. 2006;33:233–239. - PubMed
-
- Shimizu Y, Yasui K, Matsueda K, Yanagisawa A, Yamao K. Small carcinoma of the pancreas is curable: New computed tomography finding, pathological study and postoperative results from a single institute. J Gastroenterol Hepatol. 2005;20:1591–1594. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

