Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma
- PMID: 21629227
- PMCID: PMC3182345
- DOI: 10.1038/mt.2011.100
Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma
Abstract
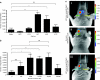
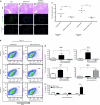
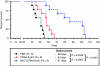
The potential utility of oncolytic adenoviruses as anticancer agents is significantly hampered by the inability of the currently available viral vectors to effectively target micrometastatic tumor burden. Neural stem cells (NSCs) have the ability to function as cell carriers for targeted delivery of an oncolytic adenovirus because of their inherent tumor-tropic migratory ability. We have previously reported that in vivo delivery of CRAd-S-pk7, a glioma-restricted oncolytic adenovirus, can enhance the survival of animals with experimental glioma. In this study, we show that intratumoral delivery of NSCs loaded with the CRAD-S-pk7 in an orthotopic xenograft model of human glioma is able to not only inhibit tumor growth but more importantly to increase median survival by ~50% versus animals treated with CRAd-S-pk7 alone (P = 0.0007). We also report that oncolytic virus infection upregulates different chemoattractant receptors and significantly enhances migratory capacity of NSCs both in vitro and in vivo. Our data further suggest that NSC-based carriers have the potential to improve the clinical efficacy of antiglioma virotherapy by not only protecting therapeutic virus from the host immune system, but also amplifying the therapeutic payload selectively at tumor sites.
Figures


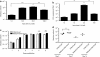
References
-
- Deorah S, Lynch CF, Sibenaller ZA., and, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2006;20:E1. - PubMed
-
- Fisher K. Striking out at disseminated metastases: the systemic delivery of oncolytic viruses. Curr Opin Mol Ther. 2006;8:301–313. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

