Novel mutation in the AVPR2 gene in a Danish male with nephrogenic diabetes insipidus caused by ER retention and subsequent lysosomal degradation of the mutant receptor
- PMID: 21629670
- PMCID: PMC3103721
- DOI: 10.1093/ndtplus/sfr010
Novel mutation in the AVPR2 gene in a Danish male with nephrogenic diabetes insipidus caused by ER retention and subsequent lysosomal degradation of the mutant receptor
Abstract
Mutations in the arginine vasopressin receptor 2 (AVPR2) gene can cause X-linked nephrogenic diabetes insipidus (NDI) characterized by the production of large amounts of urine and an inability to concentrate urine in response to the antidiuretic hormone vasopressin. We have identified a novel mutation in the AVPR2 gene (L170P) located in the fourth transmembrane domain in a Danish NDI male. Analysis of the mutant receptor in Madin-Darby Canine Kidney cell culture revealed that AVPR2-L170P was retained in the endoplasmic reticulum, and the expression was dramatically downregulated compared to wild-type AVPR2. Inhibition of the lysosome resulted in increased intracellular accumulation of AVPR2-L170P, indicating that AVPR2-L170P is downregulated via the lysosome. Inhibition of the proteasome resulted in plasma membrane localization of AVPR2-L170P, although the overall levels of AVPR2-L170P were unchanged.
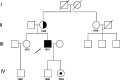
Figures



References
-
- Fujiwara TM, Bichet DG. Molecular biology of hereditary diabetes insipidus. J Am Soc Nephrol. 2005;16:2836–2846. - PubMed
-
- Robertson GL. Antidiuretic hormone. Normal and disordered function. Endocrinol Metab Clin North Am. 2001;30:671–694. vii. - PubMed
-
- Spanakis E, Milord E, Gragnoli C. AVPR2 variants and mutations in nephrogenic diabetes insipidus: review and missense mutation significance. J Cell Physiol. 2008;217:605–617. - PubMed
-
- Kim GH, Lee JW, OH YK, et al. Antidiuretic. effect of hydrochlorothiazide in lithium-induced nephrogenic diabetes insipidus is associated with upregulation of aquaporin-2, Na-Cl co-transporter, and epithelial sodium channel. J Am Soc Nephrol. 2004;15:2836–2843. - PubMed
-
- Nomura Y, Onigata K, Nagashima T, et al. Detection of skewed X-inactivation in two female carriers of vasopressin type 2 receptor gene mutation. J Clin Endocrinol Metab. 1997;82:3434–3437. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
