Poor regenerative outcome after skeletal muscle necrosis induced by Bothrops asper venom: alterations in microvasculature and nerves
- PMID: 21629691
- PMCID: PMC3101212
- DOI: 10.1371/journal.pone.0019834
Poor regenerative outcome after skeletal muscle necrosis induced by Bothrops asper venom: alterations in microvasculature and nerves
Erratum in
- PLoS One. 2011;6(7). doi: 10.1371/annotation/9caa2be8-b5e6-4553-8575-f0b575442172
Abstract
Background: Viperid snakebite envenoming is characterized by prominent local tissue damage, including muscle necrosis. A frequent outcome of such local pathology is deficient skeletal muscle regeneration, which causes muscle dysfunction, muscle loss and fibrosis, thus provoking permanent sequelae that greatly affect the quality of life of patients. The causes of such poor regenerative outcome of skeletal muscle after viperid snakebites are not fully understood.
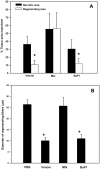
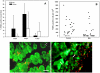
Methodology/principal findings: A murine model of muscle necrosis and regeneration was adapted to study the effects of the venom and isolated toxins of Bothrops asper, the medically most important snake in Central America. Gastrocnemius muscle was injected with either B. asper venom, a myotoxic phospholipase A(2) (Mtx), a hemorrhagic metalloproteinase (SVMP), or saline solution. At various time intervals, during one month, tissue samples were collected and analyzed by histology, and by immunocytochemical and immunohistochemical techniques aimed at detecting muscle fibers, collagen, endothelial cells, myoblasts, myotubes, macrophages, TUNEL-positive nuclei, and axons. A successful regenerative response was observed in muscle injected with Mtx, which induces myonecrosis but does not affect the microvasculature. In contrast, poor regeneration, with fibrosis and atrophic fibers, occurred when muscle was injected with venom or SVMP, both of which provoke necrosis, microvascular damage leading to hemorrhage, and poor axonal regeneration.
Conclusions/significance: The deficient skeletal muscle regeneration after injection of B. asper venom is likely to depend on the widespread damage to the microvasculature, which affects the removal of necrotic debris by phagocytes, and the provision of nutrients and oxygen required for regeneration. In addition, deficient axonal regeneration is likely to contribute to the poor regenerative outcome in this model.
Conflict of interest statement
Figures



References
-
- World Health Organization. A Neglected Public Health Issue. WHO, Geneva; 2007. Rabies and Envenomings.
-
- Fan HW, Cardoso JL. Clinical toxicology of snakebites in South America. Meier J, White J, editors. Handbook of Clinical Toxicology of Animal Venoms and Poisons. Boca Raton: CRC Press. 1995:667–688.
-
- Gutiérrez JM. Snakebite envenomation in Central America. Mackessy SP, editor. Handbook of Venoms and Toxins of Reptiles. Boca Raton: CRC Press. 2010:491–507.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

