Three-dimensional reconstruction of serial mouse brain sections: solution for flattening high-resolution large-scale mosaics
- PMID: 21629828
- PMCID: PMC3096995
- DOI: 10.3389/fnana.2011.00017
Three-dimensional reconstruction of serial mouse brain sections: solution for flattening high-resolution large-scale mosaics
Abstract
Recent advances in high-throughput technology facilitate massive data collection and sharing, enabling neuroscientists to explore the brain across a large range of spatial scales. One such form of high-throughput data collection is the construction of large-scale mosaic volumes using light microscopy (Chow et al., 2006; Price et al., 2006). With this technology, researchers can collect and analyze high-resolution digitized volumes of whole brain sections down to 0.2 μm. However, until recently, scientists lacked the tools to easily handle these large high-resolution datasets. Furthermore, artifacts resulting from specimen preparation limited volume reconstruction using this technique to only a single tissue section. In this paper, we carefully describe the steps we used to digitally reconstruct a series of consecutive mouse brain sections labeled with three stains, a stain for blood vessels (DiI), a nuclear stain (TO-PRO-3), and a myelin stain (FluoroMyelin). These stains label important neuroanatomical landmarks that are used for stacking the serial sections during reconstruction. In addition, we show that the use of two software applications, ir-Tweak and Mogrifier, in conjunction with a volume flattening procedure enable scientists to adeptly work with digitized volumes despite tears and thickness variations within tissue sections. These applications make processing large-scale brain mosaics more efficient. When used in combination with new database resources, these brain maps should allow researchers to extend the lifetime of their specimens indefinitely by preserving them in digital form, making them available for future analyses as our knowledge in the field of neuroscience continues to expand.
Keywords: cell centered database; confocal microscopy; connectivity; myelin; neuroinformatics; serial sections; warping correction; whole brain catalog.
Figures



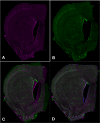


References
-
- Anderson J. R., Jones B. W., Yang J. H., Shaw M. V., Watt C. B., Koshevoy P., Spaltenstein J., Jurrus E., Kannan U. V., Whitaker R. T., Mastronarde D., Tasdizen T., Marc R. E. (2009). A computational framework for ultrastructural mapping of neural circuitry. PLoS Biol. 7, e1000074.10.1371/journal.pbio.1000074 - DOI - PMC - PubMed
-
- Belongie S. (2010). “Thin plate spline,” in From MathWorld – A Wolfram Web Resource, ed. Weisstein E. W. Available at: http://mathworld.wolfram.com/ThinPlateSpline.html
-
- Braumann U. D., Scherf N., Einenkel J., Horn L. C., Wentzensen N., Loeffler M., Kuska J. P. (2007). Large histological serial sections for computational tissue volume reconstruction. Methods Inf. Med. 46, 614–622 - PubMed
-
- Capek M., Bruza P., Janacek J., Karen P., Kubinova L., Vagnerova R. (2009). Volume reconstruction of large tissue specimens from serial physical sections using confocal microscopy and correction of cutting deformations by elastic registration. Microsc. Res. Tech. 72, 110–119 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

