Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma
- PMID: 21632504
- PMCID: PMC3139394
- DOI: 10.1200/JCO.2010.33.3005
Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma
Abstract
Purpose: Vaccination with hybridoma-derived autologous tumor immunoglobulin (Ig) idiotype (Id) conjugated to keyhole limpet hemocyanin (KLH) and administered with granulocyte-monocyte colony-stimulating factor (GM-CSF) induces follicular lymphoma (FL) -specific immune responses. To determine the clinical benefit of this vaccine, we conducted a double-blind multicenter controlled phase III trial.
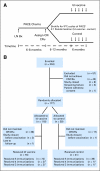
Patients and methods: Treatment-naive patients with advanced stage FL achieving complete response (CR) or CR unconfirmed (CRu) after chemotherapy were randomly assigned two to one to receive either Id vaccine (Id-KLH + GM-CSF) or control (KLH + GM-CSF). Primary efficacy end points were disease-free survival (DFS) for all randomly assigned patients and DFS for randomly assigned patients receiving at least one dose of Id vaccine or control.
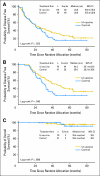
Results: Of 234 patients enrolled, 177 (81%) achieved CR/CRu after chemotherapy and were randomly assigned. For 177 randomly assigned patients, including 60 patients not vaccinated because of relapse (n = 55) or other reasons (n = 5), median DFS between Id-vaccine and control arms was 23.0 versus 20.6 months, respectively (hazard ratio [HR], 0.81; 95% CI, 0.56 to 1.16; P = .256). For 117 patients who received Id vaccine (n = 76) or control (n = 41), median DFS after randomization was 44.2 months for Id-vaccine arm versus 30.6 months for control arm (HR, 0.62; 95% CI, 0.39 to 0.99; P = .047) at median follow-up of 56.6 months (range, 12.6 to 89.3 months). In an unplanned subgroup analysis, median DFS was significantly prolonged for patients receiving IgM-Id (52.9 v 28.7 months; P = .001) but not IgG-Id vaccine (35.1 v 32.4 months; P = .807) compared with isotype-matched control-treated patients.
Conclusion: Vaccination with patient-specific hybridoma-derived Id vaccine after chemotherapy-induced CR/CRu may prolong DFS in patients with FL. Vaccine isotype may affect clinical outcome and explain differing results between this and other controlled Id-vaccine trials.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures




Comment in
-
Identifying patients with follicular lymphoma who are likely to benefit from an idiotype vaccine.J Clin Oncol. 2011 Jul 10;29(20):2748-9. doi: 10.1200/JCO.2011.35.8812. Epub 2011 May 31. J Clin Oncol. 2011. PMID: 21632499 No abstract available.
References
-
- A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma: The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–3918. - PubMed
-
- Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. - PubMed
-
- Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26:4579–4586. - PubMed
-
- Stevenson GT, Stevenson FK. Antibody to a molecularly-defined antigen confined to a tumour cell surface. Nature. 1975;254:714–716. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

