Phylogenetic differences of mammalian basal metabolic rate are not explained by mitochondrial basal proton leak
- PMID: 21632624
- PMCID: PMC3223658
- DOI: 10.1098/rspb.2011.0881
Phylogenetic differences of mammalian basal metabolic rate are not explained by mitochondrial basal proton leak
Abstract
Metabolic rates of mammals presumably increased during the evolution of endothermy, but molecular and cellular mechanisms underlying basal metabolic rate (BMR) are still not understood. It has been established that mitochondrial basal proton leak contributes significantly to BMR. Comparative studies among a diversity of eutherian mammals showed that BMR correlates with body mass and proton leak. Here, we studied BMR and mitochondrial basal proton leak in liver of various marsupial species. Surprisingly, we found that the mitochondrial proton leak was greater in marsupials than in eutherians, although marsupials have lower BMRs. To verify our finding, we kept similar-sized individuals of a marsupial opossum (Monodelphis domestica) and a eutherian rodent (Mesocricetus auratus) species under identical conditions, and directly compared BMR and basal proton leak. We confirmed an approximately 40 per cent lower mass specific BMR in the opossum although its proton leak was significantly higher (approx. 60%). We demonstrate that the increase in BMR during eutherian evolution is not based on a general increase in the mitochondrial proton leak, although there is a similar allometric relationship of proton leak and BMR within mammalian groups. The difference in proton leak between endothermic groups may assist in elucidating distinct metabolic and habitat requirements that have evolved during mammalian divergence.
Figures
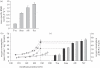




References
-
- Bininda-Emonds O. R. P., et al. 2008. The delayed rise of present-day mammals. Nature 456, 274.10.1038/nature07347 (doi:10.1038/nature07347) - DOI - DOI - PubMed
-
- Dawson T. J., Hulbert A. J. 1970. Standard metabolism, body temperature, and surface areas of Australian marsupials. Am. J. Physiol. 218, 1233–1238 - PubMed
-
- Frappell P. B., Butler P. J. 2004. Minimal metabolic rate, what it is, its usefulness, and its relationship to the evolution of endothermy: a brief synopsis. Physiol. Biochem. Zool. 77, 865–86810.1086/425191 (doi:10.1086/425191) - DOI - DOI - PubMed
-
- Kleiber M. 1961. Body size and metabolic rate. The fire of life (ed. Kleiber M.) pp. 177–216 New York, NY: Wiley
-
- Hemmingsen A. M. 1960. Energy metabolism as related to body size and respiratory surfaces, and its evolution. Report Stemo. Meml. Ho. 9, 1–110
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

